Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs
- PMID: 25317669
- PMCID: PMC4491130
- DOI: 10.1165/rcmb.2014-0250OC
Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs
Abstract
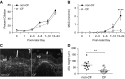
Mucociliary clearance (MCC) and submucosal glands are major components of airway innate immunity that have impaired function in cystic fibrosis (CF). Although both of these defense systems develop postnatally in the ferret, the lungs of newborn ferrets remain sterile in the presence of a functioning cystic fibrosis transmembrane conductance regulator gene. We evaluated several components of airway innate immunity and inflammation in the early CF ferret lung. At birth, the rates of MCC did not differ between CF and non-CF animals, but the height of the airway surface liquid was significantly reduced in CF newborn ferrets. CF ferrets had impaired MCC after 7 days of age, despite normal rates of ciliogenesis. Only non-CF ferrets eradicated Pseudomonas directly introduced into the lung after birth, whereas both genotypes could eradicate Staphylococcus. CF bronchoalveolar lavage fluid (BALF) had significantly lower antimicrobial activity selectively against Pseudomonas than non-CF BALF, which was insensitive to changes in pH and bicarbonate. Liquid chromatography-tandem mass spectrometry and cytokine analysis of BALF from sterile Caesarean-sectioned and nonsterile naturally born animals demonstrated CF-associated disturbances in IL-8, TNF-α, and IL-β, and pathways that control immunity and inflammation, including the complement system, macrophage functions, mammalian target of rapamycin signaling, and eukaryotic initiation factor 2 signaling. Interestingly, during the birth transition, IL-8 was selectively induced in CF BALF, despite no genotypic difference in bacterial load shortly after birth. These results suggest that newborn CF ferrets have defects in both innate immunity and inflammatory signaling that may be important in the early onset and progression of lung disease in these animals.
Keywords: animal model; cystic fibrosis; cystic fibrosis transmembrane conductance regulator; inflammation; innate immunity.
Figures





References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. - PubMed
-
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. - PubMed
-
- Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK047967/DK/NIDDK NIH HHS/United States
- P30 DK072482/DK/NIDDK NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- HL108902/HL/NHLBI NIH HHS/United States
- DK072482/DK/NIDDK NIH HHS/United States
- R01 HL108902/HL/NHLBI NIH HHS/United States
- R37 DK047967/DK/NIDDK NIH HHS/United States
- R01 DK047967/DK/NIDDK NIH HHS/United States
- R24 HL123482/HL/NHLBI NIH HHS/United States
- DK054759/DK/NIDDK NIH HHS/United States
- HL1116213/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- R01 HL116213/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

