Chimeric antigen receptor T cells for sustained remissions in leukemia
- PMID: 25317870
- PMCID: PMC4267531
- DOI: 10.1056/NEJMoa1407222
Chimeric antigen receptor T cells for sustained remissions in leukemia
Erratum in
-
Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia; Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia; Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia.N Engl J Med. 2016 Mar 10;374(10):998. doi: 10.1056/NEJMx160005. N Engl J Med. 2016. PMID: 26962747 No abstract available.
Abstract
Background: Relapsed acute lymphoblastic leukemia (ALL) is difficult to treat despite the availability of aggressive therapies. Chimeric antigen receptor-modified T cells targeting CD19 may overcome many limitations of conventional therapies and induce remission in patients with refractory disease.
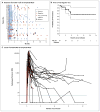
Methods: We infused autologous T cells transduced with a CD19-directed chimeric antigen receptor (CTL019) lentiviral vector in patients with relapsed or refractory ALL at doses of 0.76×10(6) to 20.6×10(6) CTL019 cells per kilogram of body weight. Patients were monitored for a response, toxic effects, and the expansion and persistence of circulating CTL019 T cells.
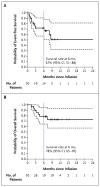
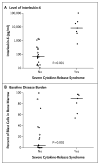
Results: A total of 30 children and adults received CTL019. Complete remission was achieved in 27 patients (90%), including 2 patients with blinatumomab-refractory disease and 15 who had undergone stem-cell transplantation. CTL019 cells proliferated in vivo and were detectable in the blood, bone marrow, and cerebrospinal fluid of patients who had a response. Sustained remission was achieved with a 6-month event-free survival rate of 67% (95% confidence interval [CI], 51 to 88) and an overall survival rate of 78% (95% CI, 65 to 95). At 6 months, the probability that a patient would have persistence of CTL019 was 68% (95% CI, 50 to 92) and the probability that a patient would have relapse-free B-cell aplasia was 73% (95% CI, 57 to 94). All the patients had the cytokine-release syndrome. Severe cytokine-release syndrome, which developed in 27% of the patients, was associated with a higher disease burden before infusion and was effectively treated with the anti-interleukin-6 receptor antibody tocilizumab.
Conclusions: Chimeric antigen receptor-modified T-cell therapy against CD19 was effective in treating relapsed and refractory ALL. CTL019 was associated with a high remission rate, even among patients for whom stem-cell transplantation had failed, and durable remissions up to 24 months were observed. (Funded by Novartis and others; CART19 ClinicalTrials.gov numbers, NCT01626495 and NCT01029366.).
Figures

Comment in
-
Immunotherapy: CAR-modified T cells targeting CD19-curing the incurable.Nat Rev Clin Oncol. 2014 Dec;11(12):683. doi: 10.1038/nrclinonc.2014.187. Epub 2014 Oct 28. Nat Rev Clin Oncol. 2014. PMID: 25348787 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
