Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials
- PMID: 25318069
- PMCID: PMC4197780
- DOI: 10.7554/eLife.03695
Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials
Abstract
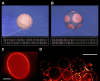
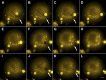
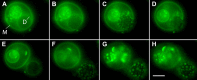
Giant lipid vesicles are closed compartments consisting of semi-permeable shells, which isolate femto- to pico-liter quantities of aqueous core from the bulk. Although water permeates readily across vesicular walls, passive permeation of solutes is hindered. In this study, we show that, when subject to a hypotonic bath, giant vesicles consisting of phase separating lipid mixtures undergo osmotic relaxation exhibiting damped oscillations in phase behavior, which is synchronized with swell-burst lytic cycles: in the swelled state, osmotic pressure and elevated membrane tension due to the influx of water promote domain formation. During bursting, solute leakage through transient pores relaxes the pressure and tension, replacing the domain texture by a uniform one. This isothermal phase transition--resulting from a well-coordinated sequence of mechanochemical events--suggests a complex emergent behavior allowing synthetic vesicles produced from simple components, namely, water, osmolytes, and lipids to sense and regulate their micro-environment.
Keywords: biophysics; cell biology; compartmentalization; giant phospholipid vesicles; lipid rafts; phase separation; primitive osmoregulation; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







Similar articles
-
Mixing Water, Transducing Energy, and Shaping Membranes: Autonomously Self-Regulating Giant Vesicles.Langmuir. 2016 Mar 8;32(9):2151-63. doi: 10.1021/acs.langmuir.5b04470. Epub 2016 Feb 11. Langmuir. 2016. PMID: 26866787
-
Pulsatile Lipid Vesicles under Osmotic Stress.Biophys J. 2017 Apr 25;112(8):1682-1691. doi: 10.1016/j.bpj.2017.03.018. Biophys J. 2017. PMID: 28445759 Free PMC article.
-
Permeability and Line-Tension-Dependent Response of Polyunsaturated Membranes to Osmotic Stresses.Biophys J. 2018 Nov 20;115(10):1942-1955. doi: 10.1016/j.bpj.2018.09.031. Epub 2018 Oct 6. Biophys J. 2018. PMID: 30366629 Free PMC article.
-
Elementary Processes and Mechanisms of Interactions of Antimicrobial Peptides with Membranes-Single Giant Unilamellar Vesicle Studies.Adv Exp Med Biol. 2019;1117:17-32. doi: 10.1007/978-981-13-3588-4_3. Adv Exp Med Biol. 2019. PMID: 30980351 Review.
-
Giant Vesicles and Their Use in Assays for Assessing Membrane Phase State, Curvature, Mechanics, and Electrical Properties.Annu Rev Biophys. 2019 May 6;48:93-119. doi: 10.1146/annurev-biophys-052118-115342. Epub 2019 Feb 27. Annu Rev Biophys. 2019. PMID: 30811220 Review.
Cited by
-
Visualizing Tension and Growth in Model Membranes Using Optical Dyes.Biophys J. 2018 Oct 2;115(7):1307-1315. doi: 10.1016/j.bpj.2018.08.021. Epub 2018 Aug 27. Biophys J. 2018. PMID: 30219285 Free PMC article.
-
Beating Vesicles: Encapsulated Protein Oscillations Cause Dynamic Membrane Deformations.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16286-16290. doi: 10.1002/anie.201808750. Epub 2018 Nov 20. Angew Chem Int Ed Engl. 2018. PMID: 30270475 Free PMC article.
-
HydroFlipper membrane tension probes: imaging membrane hydration and mechanical compression simultaneously in living cells.Chem Sci. 2022 Feb 3;13(7):2086-2093. doi: 10.1039/d1sc05208j. eCollection 2022 Feb 16. Chem Sci. 2022. PMID: 35308858 Free PMC article.
-
Intelligent fluorescence image analysis of giant unilamellar vesicles using convolutional neural network.BMC Bioinformatics. 2022 Jan 21;23(1):48. doi: 10.1186/s12859-022-04577-2. BMC Bioinformatics. 2022. PMID: 35062867 Free PMC article.
-
Physical Concept to Explain the Regulation of Lipid Membrane Phase Separation under Isothermal Conditions.Life (Basel). 2023 Apr 28;13(5):1105. doi: 10.3390/life13051105. Life (Basel). 2023. PMID: 37240749 Free PMC article. Review.
References
-
- Angelova MI, Soléau S, Méléard P, Faucon JE, Bothorel P. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progress in Colloid and Polymer Science 89:127–131. doi: 10.1007/BFb0116295 - DOI

