CD39 improves survival in microbial sepsis by attenuating systemic inflammation
- PMID: 25318479
- PMCID: PMC4285550
- DOI: 10.1096/fj.14-253567
CD39 improves survival in microbial sepsis by attenuating systemic inflammation
Abstract
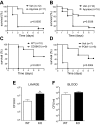
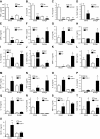
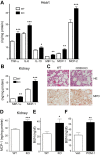
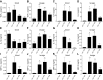
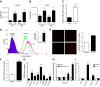
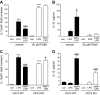
Sepsis remains the leading cause of morbidity and mortality in critically ill patients. Excessive inflammation is a major cause of organ failure and mortality in sepsis. Ectonucleoside triphosphate diphosphohydrolase 1, ENTPDase1 (CD39) is a cell surface nucleotide-metabolizing enzyme, which degrades the extracellular purines ATP and ADP, thereby regulating purinergic receptor signaling. Although the role of purinergic receptor signaling in regulating inflammation and sepsis has been addressed previously, the role of CD39 in regulating the host's response to sepsis is unknown. We found that the CD39 mimic apyrase (250 U/kg) decreased and knockout or pharmacologic blockade with sodium polyoxotungstate (5 mg/kg; IC50 ≈ 10 μM) of CD39 increased mortality of mice with polymicrobial sepsis induced by cecal ligation and puncture. CD39 decreased inflammation, organ damage, immune cell apoptosis, and bacterial load. Use of bone marrow chimeric mice revealed that CD39 expression on myeloid cells decreases inflammation in septic mice. CD39 expression is upregulated during sepsis in mice, as well as in both murine and human macrophages stimulated with Escherichia coli. Moreover, E. coli increases CD39 promoter activity in macrophages. Altogether, these data indicate CD39 as an evolutionarily conserved inducible protective pathway during sepsis. We propose CD39 as a novel therapeutic target in the management of sepsis.
Keywords: MIP; TNF; interleukin; kidney; lung.
© FASEB.
Figures
References
-
- Van der Poll T., and Opal S. M. (2008) Host-pathogen interactions in sepsis. Lancet Infect. Dis. 8, 32–43 - PubMed
-
- Bone R. C., Balk R. A., Cerra F. B., Dellinger R. P., Fein A. M., Knaus W. A., Schein R. M., and Sibbald W. J.; ACCP/SCCM Consensus Conference Committee (2009) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest 136(5, Suppl)e28. - PubMed
-
- Robertson C. M., and Coopersmith C. M. (2006) The systemic inflammatory response syndrome. Microbes Infect. 8, 1382–1389 - PubMed
-
- Xiao W., Mindrinos M. N., Seok J., Cuschieri J., Cuenca A. G., Gao H., Hayden D. L., Hennessy L., Moore E. E., Minei J. P., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Brownstein B. H., Mason P. H., Baker H. V., Finnerty C. C., Jeschke M. G., López M. C., Klein M. B., Gamelli R. L., Gibran N. S., Arnoldo B., Xu W., Zhang Y., Calvano S. E., McDonald-Smith G. P., Schoenfeld D. A., Storey J. D., Cobb J. P., Warren H. S., Moldawer L. L., Herndon D. N., Lowry S. F., Maier R. V., Davis R. W., and Tompkins R. G.; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program (2011) A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

