Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation
- PMID: 25318534
- PMCID: PMC4215016
- DOI: 10.1186/s12989-014-0048-2
Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation
Abstract
Background: Carbon nanotubes (CNT) represent a great promise for technological and industrial development but serious concerns on their health effects have also emerged. Rod-shaped CNT are, in fact, able to induce asbestos-like pathogenicity in mice including granuloma formation in abdominal cavity and sub-pleural fibrosis. Exposure to CNT, especially in the occupational context, happens mainly by inhalation. However, little is known about the possible effects of CNT on pulmonary allergic diseases, such as asthma.
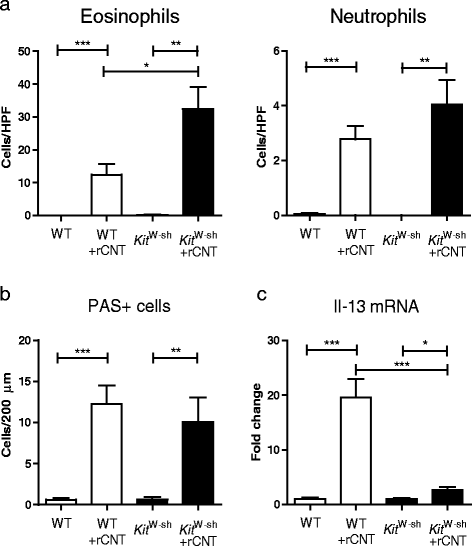
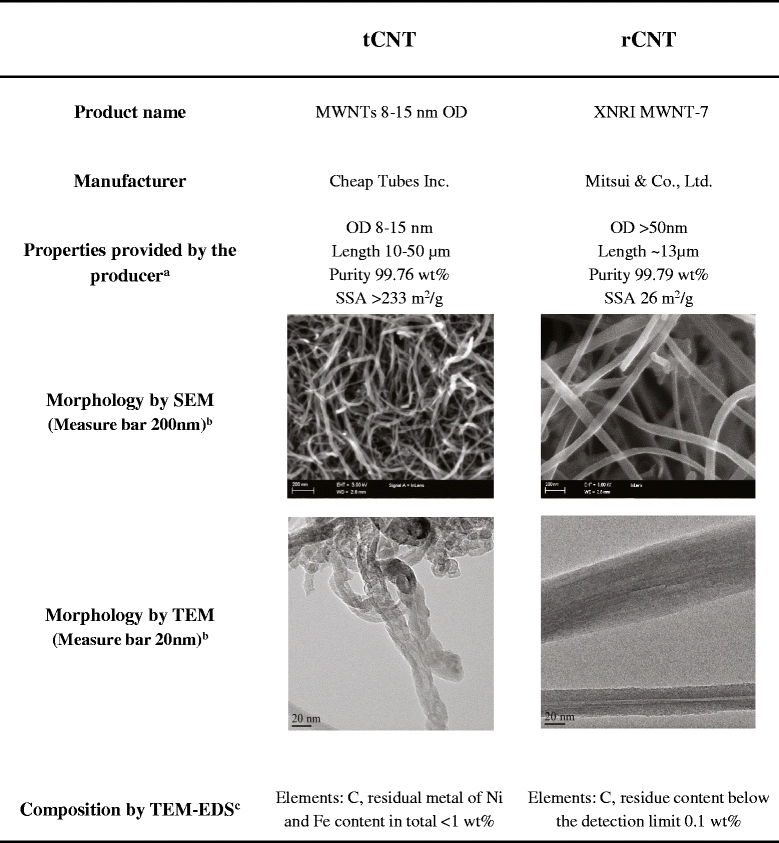
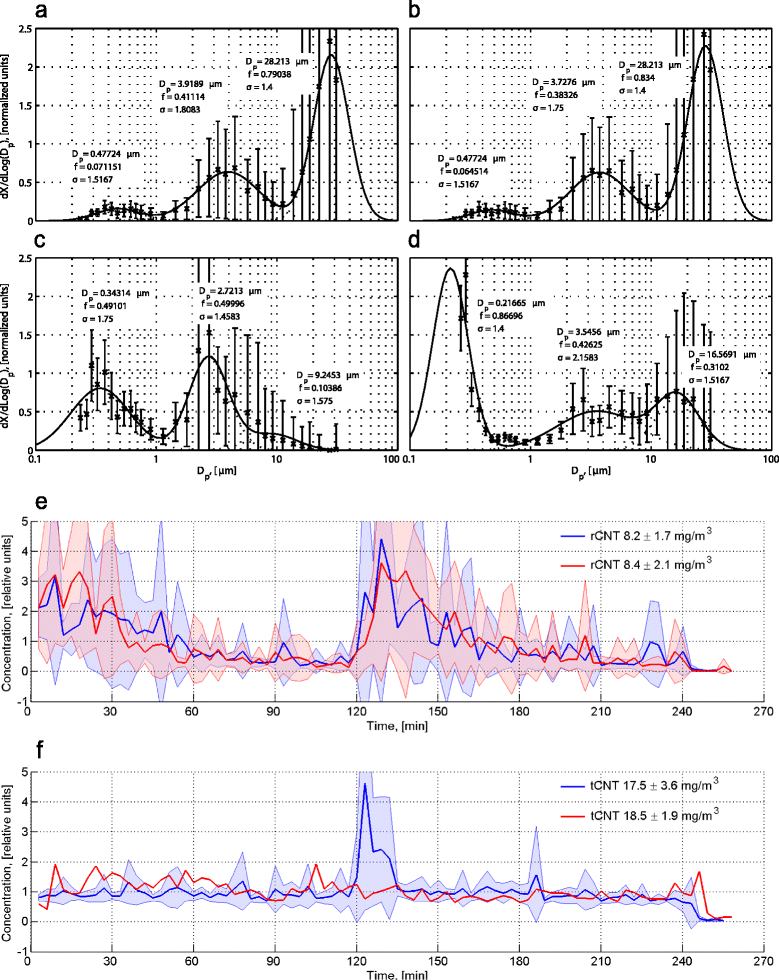
Methods: We exposed mice by inhalation to two types of multi-walled CNT, rigid rod-like and flexible tangled CNT, for four hours a day once or on four consecutive days. Early events were monitored immediately and 24 hours after the single inhalation exposure and the four day exposure mimicked an occupational work week. Mast cell deficient mice were used to evaluate the role of mast cells in the occurring inflammation.
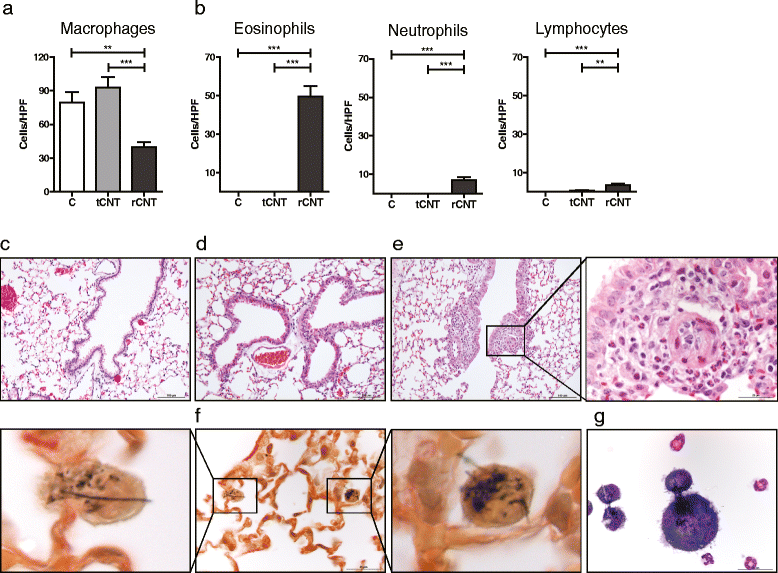
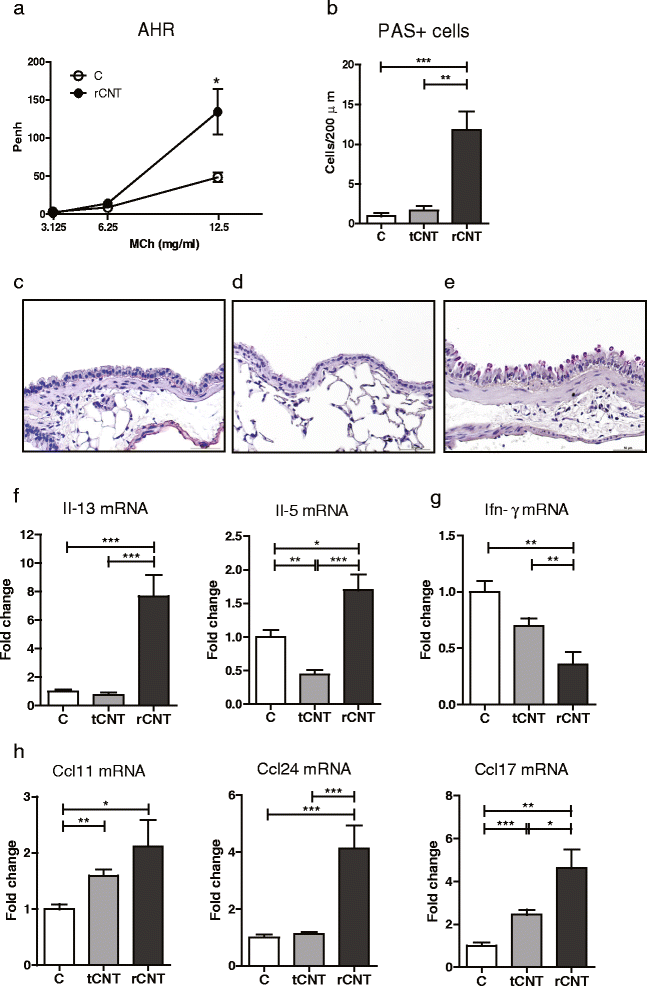
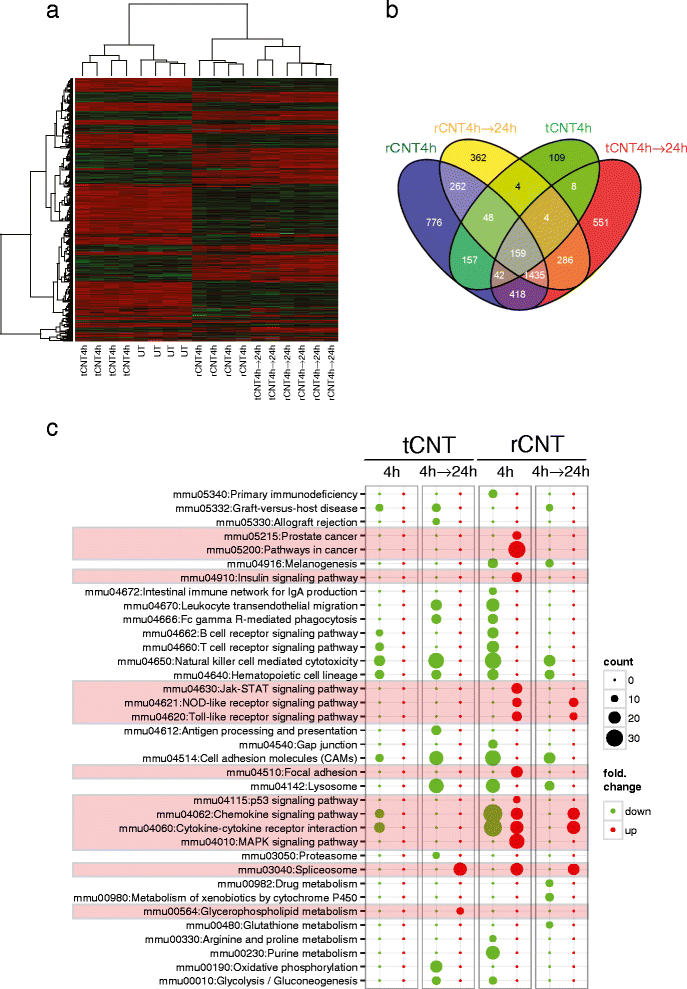
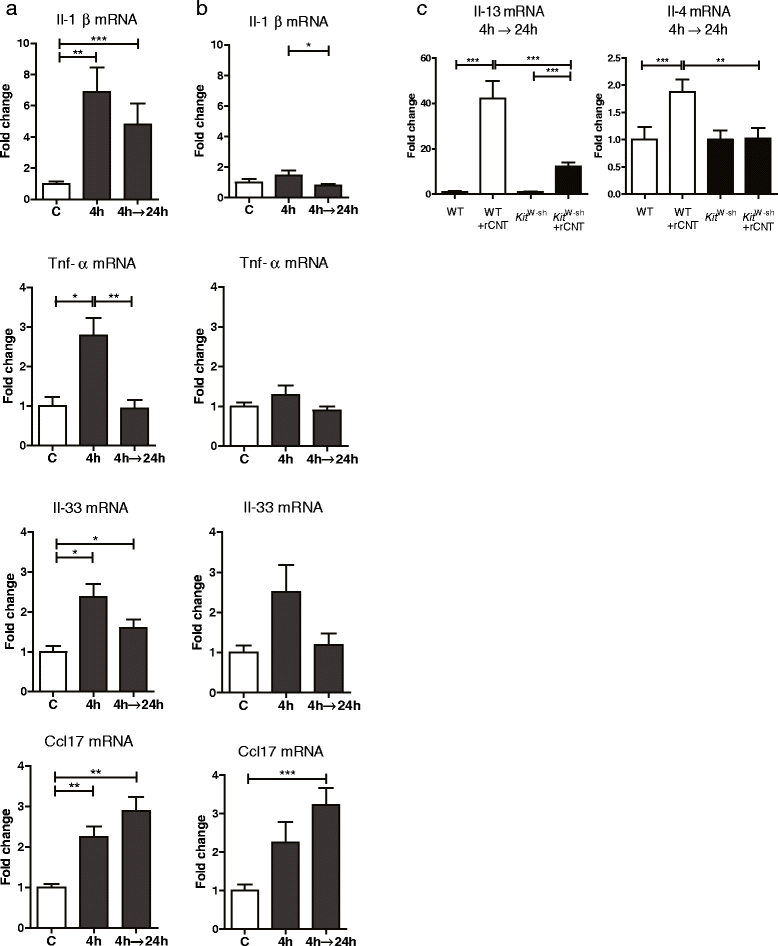
Results: Here we show that even a short-term inhalation of the rod-like CNT induces novel innate immunity-mediated allergic-like airway inflammation in healthy mice. Marked eosinophilia was accompanied by mucus hypersecretion, AHR and the expression of Th2-type cytokines. Exploration of the early events by transcriptomics analysis reveals that a single 4-h exposure to rod-shaped CNT, but not to tangled CNT, causes a radical up-regulation of genes involved in innate immunity and cytokine/chemokine pathways. Mast cells were found to partially regulate the inflammation caused by rod-like CNT, but also alveaolar macrophages play an important role in the early stages.
Conclusions: These observations emphasize the diverse abilities of CNT to impact the immune system, and they should be taken into account for hazard assessment.
Figures
Similar articles
-
A Single Aspiration of Rod-like Carbon Nanotubes Induces Asbestos-like Pulmonary Inflammation Mediated in Part by the IL-1 Receptor.Toxicol Sci. 2015 Sep;147(1):140-55. doi: 10.1093/toxsci/kfv112. Epub 2015 Jun 5. Toxicol Sci. 2015. PMID: 26048651
-
Long-term polarization of alveolar macrophages to a profibrotic phenotype after inhalation exposure to multi-wall carbon nanotubes.PLoS One. 2018 Oct 29;13(10):e0205702. doi: 10.1371/journal.pone.0205702. eCollection 2018. PLoS One. 2018. PMID: 30372450 Free PMC article.
-
Atomic layer deposition coating of carbon nanotubes with zinc oxide causes acute phase immune responses in human monocytes in vitro and in mice after pulmonary exposure.Part Fibre Toxicol. 2016 Jun 8;13(1):29. doi: 10.1186/s12989-016-0141-9. Part Fibre Toxicol. 2016. PMID: 27278808 Free PMC article.
-
A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks.Crit Rev Toxicol. 2006 Mar;36(3):189-217. doi: 10.1080/10408440600570233. Crit Rev Toxicol. 2006. PMID: 16686422 Review.
-
Pulmonary toxicity of carbon nanotubes and asbestos - similarities and differences.Adv Drug Deliv Rev. 2013 Dec;65(15):2078-86. doi: 10.1016/j.addr.2013.07.014. Epub 2013 Jul 27. Adv Drug Deliv Rev. 2013. PMID: 23899865 Review.
Cited by
-
Physicochemical predictors of Multi-Walled Carbon Nanotube-induced pulmonary histopathology and toxicity one year after pulmonary deposition of 11 different Multi-Walled Carbon Nanotubes in mice.Basic Clin Pharmacol Toxicol. 2019 Feb;124(2):211-227. doi: 10.1111/bcpt.13119. Epub 2018 Oct 18. Basic Clin Pharmacol Toxicol. 2019. PMID: 30168672 Free PMC article.
-
Characterization of ENM Dynamic Dose-Dependent MOA in Lung with Respect to Immune Cells Infiltration.Nanomaterials (Basel). 2022 Jun 13;12(12):2031. doi: 10.3390/nano12122031. Nanomaterials (Basel). 2022. PMID: 35745370 Free PMC article.
-
Advanced Functional Materials Based on Nanocellulose for Pharmaceutical/Medical Applications.Pharmaceutics. 2021 Jul 23;13(8):1125. doi: 10.3390/pharmaceutics13081125. Pharmaceutics. 2021. PMID: 34452086 Free PMC article. Review.
-
Nano-risk Science: application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes.Part Fibre Toxicol. 2016 Mar 15;13:15. doi: 10.1186/s12989-016-0125-9. Part Fibre Toxicol. 2016. PMID: 26979667 Free PMC article.
-
Signaling Pathways Implicated in Carbon Nanotube-Induced Lung Inflammation.Front Immunol. 2020 Dec 11;11:552613. doi: 10.3389/fimmu.2020.552613. eCollection 2020. Front Immunol. 2020. PMID: 33391253 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

