Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies
- PMID: 25318966
- PMCID: PMC6703418
- DOI: 10.1002/14651858.CD005979.pub2
Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies
Abstract
Background: Pharmaceuticals are important interventions that could improve people's health. Pharmaceutical pricing and purchasing policies are used as cost-containment measures to determine or affect the prices that are paid for drugs. Internal reference pricing establishes a benchmark or reference price within a country which is the maximum level of reimbursement for a group of drugs. Other policies include price controls, maximum prices, index pricing, price negotiations and volume-based pricing.
Objectives: To determine the effects of pharmaceutical pricing and purchasing policies on health outcomes, healthcare utilisation, drug expenditures and drug use.
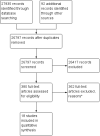
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), part of The Cochrane Library (including the Effective Practice and Organisation of Care Group Register) (searched 22/10/2012); MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE, Ovid (searched 22/10/2012); EconLit, ProQuest (searched 22/10/2012); PAIS International, ProQuest (searched 22/10/2012); World Wide Political Science Abstracts, ProQuest (searched 22/10/2012); INRUD Bibliography (searched 22/10/2012); Embase, Ovid (searched 14/12/2010); NHSEED, part of The Cochrane Library (searched 08/12/2010); LILACS, VHL (searched 14/12/2010); International Political Science Abstracts (IPSA), Ebsco (searched (17/12/2010); OpenSIGLE (searched 21/12/10); WHOLIS, WHO (searched 17/12/2010); World Bank (Documents and Reports) (searched 21/12/2010); Jolis (searched 09/10/2011); Global Jolis (searched 09/10/2011) ; OECD (searched 30/08/2005); OECD iLibrary (searched 30/08/2005); World Bank eLibrary (searched 21/12/2010); WHO - The Essential Drugs and Medicines web site (browsed 21/12/2010).
Selection criteria: Policies in this review were defined as laws; rules; financial and administrative orders made by governments, non-government organisations or private insurers. To be included a study had to include an objective measure of at least one of the following outcomes: drug use, healthcare utilisation and health outcomes or costs (expenditures); the study had to be a randomised trial, non-randomised trial, interrupted time series (ITS), repeated measures (RM) study or a controlled before-after study of a pharmaceutical pricing or purchasing policy for a large jurisdiction or system of care.
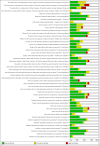
Data collection and analysis: Two review authors independently extracted data and assessed the risk of bias. Results were summarised in tables. There were too few comparisons with similar outcomes across studies to allow for meta-analysis or meaningful exploration of heterogeneity.
Main results: We included 18 studies (seven identified in the update): 17 of reference pricing, one of which also assessed maximum prices, and one of index pricing. None of the studies were trials. All included studies used ITS or RM analyses. The quality of the evidence was low or very low for all outcomes. Three reference pricing studies reported cumulative drug expenditures at one year after the transition period. Two studies reported the median relative insurer's cumulative expenditures, on both reference drugs and cost share drugs, of -18%, ranging from -36% to 3%. The third study reported relative insurer's cumulative expenditures on total market of -1.5%. Four reference pricing studies reported median relative insurer's expenditures on both reference drugs and cost share drugs of -10%, ranging from -53% to 4% at one year after the transition period. Four reference pricing studies reported a median relative change of 15% in reference drugs prescriptions at one year (range -14% to 166%). Three reference pricing studies reported a median relative change of -39% in cost share drugs prescriptions at one year (range -87% to -17%). One study of index pricing reported a relative change of 55% (95% CI 11% to 98%) in the use of generic drugs and -43% relative change (95% CI -67% to -18%) in brand drugs at six months after the transition period. The same study reported a price change of -5.3% and -1.1% for generic and brand drugs respectively six months after the start of the policy. One study of maximum prices reported a relative change in monthly sales volume of all statins of 21% (95% CI 19% to 24%) after one year of the introduction of this policy. Four studies reported effects on mortality and healthcare utilisation, however they were excluded because of study design limitations.
Authors' conclusions: The majority of the studies of pricing and purchasing policies that met our inclusion criteria evaluated reference pricing. We found that internal reference pricing may reduce expenditures in the short term by shifting drug use from cost share drugs to reference drugs. Reference pricing may reduce related expenditures with effects on reference drugs but the effect on expenditures of cost share drugs is uncertain. Reference pricing may increase the use of reference drugs and may reduce the use of cost share drugs. The analysis and reporting of the effects on patients' drug expenditures were limited in the included studies and administration costs were not reported. Reference pricing effects on health are uncertain due to lack of evidence. The effects of other purchasing and pricing policies are until now uncertain due to sparse evidence. However, index pricing may reduce the use of brand drugs, increase the use of generic drugs, and may also slightly reduce the price of the generic drug when compared with no intervention.
Conflict of interest statement
For the previous version of this review:
MOA has previously carried out short term pharmacoeconomic projects for the National Insurance Service and the Norwegian Medicines Agency. From 1997 to 1999 he worked for a private company, Brevreklame, doing market research for pharmaceutical firms in Norway. HS is supported by the Dutch Health Care Insurance Board (CVZ). JPK has previously worked for one year for each of the Danish Medicines Agency and Lundbeck A/S as part of a residency in clinical pharmacology and has been previously employed five hours a week at the Danish Medicines Agency (Licensing Division). Since March 2007 he has been working zero to five hours a week as an advisor for Nordic Biotech.
For this update:
AA has previously carried out short term pharmacoeconomic projects for the Commerce Ministry and other technical projects for the Ministry of Health and the local National Regulatory Authority (INVIMA) in Colombia.
AC has not carried out pharmacoeconomic projects before now.
Figures
Update of
-
Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD005979. doi: 10.1002/14651858.CD005979. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Oct 16;(10):CD005979. doi: 10.1002/14651858.CD005979.pub2. PMID: 16625648 Updated.
References
References to studies included in this review
Aronsson 2001 {published data only}
-
- Aronsson T, Bergman MA, Rudholm N. The impact of generic drug competition on brand name market shares ‐ evidence from micro data. Review of Industrial Organization 2001;19(4):425‐32. [MEDLINE: ]
Brekke 2003 {published data only}
-
- Brekke K, Grasdal A, Holmås TH, Steen F, Sunnevåg K. Evaluation of the new pharmacy act and the index price system [Evaluering av ny apoteklov og indeksprissystemet]. Bergen (Norway): Rokkansenteret; 2003; Rapport 17. Beregen, Norway: Rokkansenteret. [MEDLINE: ]
Brekke 2011 {published data only}
-
- Brekke KR, Holmas TH, Straume OR. Reference pricing, competition, and pharmaceutical expenditures: Theory and evidence from a natural experiment. Journal of Public Economics 2011;95:624‐38.
Grootendorst 2002 {published data only}
-
- Grootendorst P, Dolovich L, Holbrook A, Levy A, O'Brien B. The impact of reference pricing of cardiovascular drugs on health care costs and health outcomes: evidence from British Columbia. Volume 2: Technical Report. SEDAP research paper no. 71. Hamilton (ON): McMaster University QSEP Report Series No. 370; 2002. 2. Hamilton, Ontario, Canada: SEDAP.
Grootendorst 2005 {published data only}
Grootendorst 2006 {published and unpublished data}
-
- Grootendorst P, Stewart D. A re‐examination of the impact of reference pricing on anti‐hypertensive drug plan expenditures in British Columbia. Health Economics 24 February 2006;15:735–42. - PubMed
Hazlet 2002 {published data only}
-
- Hazlet TK, Blough DK. Health services utilization with reference drug pricing of histamine(2) receptor antagonists in British Columbia elderly. Medical Care 2002;40(8):640‐9. - PubMed
Kibicho 2012 {published data only}
Marshall 2002 {published data only}
McManus 2001 {published data only}
-
- McManus P, Birkett DJ, Dudley J, Stevens A. Impact of the minimum pricing policy and introduction of brand (generic) substitution into the Pharmaceutical Benefits Scheme in Australia. Pharmacoepidemiology and Drug Safety 2001;10(4):295‐300. - PubMed
Moreno‐Torres 2011 {published data only}
-
- Moreno‐Torres I, Puig‐Junoy J, Raya JM. The impact of repeated cost containment policies on pharmaceutical expenditure: Experience in Spain. European Journal of Health Economics 2011;12:563‐73. - PubMed
Narine 2001 {published data only}
-
- Narine L, Senathirajah M, Smith T. An assessment of the impact of reference‐based pricing policies on the H2 antagonist market in British Columbia, Canada. Journal of Research in Pharmaceutical Economics 2001;11(1):63‐78.
Pavcnik 2002 {published data only}
-
- Pavcnik N. Do pharmaceutical prices respond to insurance?. Cambridge (MA): National Bureau of Economic Research; Working Paper 7865; 2000.
-
- Pavcnik N. Do pharmaceutical prices respond to potential patient out‐of‐pocket expenses?. Rand Journal of Economics 2002;33(3):469‐487. [MEDLINE: ] - PubMed
Puig 2007 {published and unpublished data}
-
- Puig‐Junoy J. The impact of generic reference pricing interventions in the statin market. Health Policy 2007;84(1):14–29. - PubMed
Sawyer 1983 {published data only}
-
- Sawyer DO. Pharmaceutical reimbursement and drug cost control: the MAC experience in Maryland. Inquiry 1983;20(1):76‐87. - PubMed
Schneeweiss 2002 {published data only}
-
- Schneeweiss S, Maclure M, Soumerai SB. Prescription duration after drug copay changes in older people: methodological aspects. Journal of the American Geriatrics Society 2002;50(3):521‐5. - PubMed
-
- Schneeweiss S, Walker AM, Glynn RJ, Maclure M, Dormuth C, Soumerai SB. Outcomes of reference pricing for angiotensin‐converting‐enzyme inhibitors. New England Journal of Medicine 2002;346(11):822‐9. - PubMed
Schneeweiss 2003 {published data only}
-
- Schneeweiss S, Soumerai SB, Maclure M, Dormuth C, Walker AM, Glynn RJ. Clinical and economic consequences of reference pricing for dihydropyridine calcium channel blockers. Clinical Pharmacology and Therapeutics 2003;74(4):388‐400. - PubMed
Stargardt 2010 {published and unpublished data}
-
- Stargardt T. The impact of reference pricing on switching behaviour and healthcare utilisation: the case of statins in Germany. European Journal of Health Economics 29 July 2010;2010(11):267–77. - PubMed
References to studies excluded from this review
Anis 1994 {published data only}
-
- Anis AH. Substitution laws, insurance coverage, and generic drug use. Medical Care 1994;32(3):240‐56. - PubMed
Anis 2003 {published data only}
-
- Anis AH, Guh DP, Woolcott J. Lowering generic drug prices: less regulation equals more competition. Medical Care 2003;41(1):135‐41. [MEDLINE: ] - PubMed
Atella 2000 {published data only}
-
- Atella V. Drug cost containment policies in Italy: are they really effective in the long‐run? The case of minimum reference price. Health Policy 2000;50(3):197‐218. [MEDLINE: ] - PubMed
Barberato 2007 {published data only}
-
- Barberato FS, Lopes LC. The influence of the profit margin on the marketing of medicines. [Portuguese]. Revista de Ciencias Farmaceuticas Basica e Aplicada 2007;28(1):99‐106.
Bergman 1998 {published data only}
-
- Bergman M, Aronsson T, Rudholm N. Reduced drug costs with the reference cost system [Swedish] [Sankta lakemedelspriser med referensprissystemet]. Lakartidningen 1998;95(23):2715‐7. [MEDLINE: ] - PubMed
Bergman 2003 {published data only}
-
- Bergman MA, Rudholm N. The relative importance of actual and potential competition: empirical evidence from the pharmaceuticals market. Journal of Industrial Economics 2003;51:455‐67.
Borrell 1999 {published data only}
-
- Borrell JR. Pharmaceutical price regulation. A study on the impact of the rate‐of‐return regulation in the UK. Pharmacoeconomics 1999;15(3):291‐303. [MEDLINE: ] - PubMed
Boyce 1990 {published data only}
-
- Boyce D, Bartlett G. The maximum medical aid price programme. A review of the concept and of its ability to reduce expenditure on medicines. South African Medical Journal 1990;78:147‐51. [MEDLINE: ] - PubMed
Danzon 1997 {published data only}
-
- Danzon PM, Liu H. Reference pricing and physician drug budgets: The German experience in controlling pharmaceutical expenditures. Philadelphia (USA): Wharton School Working Paper; University of Philadelpha; 1997. Wharton School Working Paper; University of Philadelpha, 1997. [MEDLINE: ]
Duetz 2003 {published data only}
-
- Duetz MS, Schneeweiss S, Maclure M, Abel T, Glynn RJ, Soumerai SB. Physician gender and changes in drug prescribing after the implementation of reference pricing in British Columbia. Clinical Therapeutics 2003;25(1):273‐84. - PubMed
ECON 2000 {published data only}
-
- ECON. Evaluation of the reference price system for pharmaceuticals [Norwegian] [Evaluering av referanseprissystemet for legemidler]. Oslo (Norway): ECON; 2000. ECON‐rapport 44/2000; Oslo Vol. ECON‐rapport 44/2000.
Ekelund 2001 {published data only}
-
- Ekelund M. Generic entry before and after the introduction of reference prices. In: Ekelund M editor(s). Competition and innovation in the Swedish pharmaceutical market. Stockholm: Stockholm School of Economics, 2001:Chapter 4. [MEDLINE: ]
Giuliani 1998 {published data only}
-
- Giuliani G, Selke G, Garattini L. The German experience in reference pricing. Health Policy 1998;44:73‐85. - PubMed
Hsiao 2010 {published data only}
Inocencio 2010 {published data only}
-
- Marcos I, Sanches Amorim MC, Hoyos Guevara AJ, Vivo B. Financial incentives for generic drugs: case study on a reimbursement program. Einstein (Säo Paulo) 2010;8(2 Pt 1):154‐61. - PubMed
Johnson 2011 {published data only}
Lee 1983 {published data only}
Li 2008 {published data only}
-
- Li L, Chen Y, Yao L, Li Y. Evaluation of the effects of implementing the policy "Without Added Profit" to sale drug in community health service institutions of Chengdu. [Chinese]. Chinese Journal of New Drugs 2008;17(21):1820‐2,1842.
Morton 1997 {published data only}
-
- Morton FMS. The interaction between a most‐favored‐customer clause and price dispersion: an empirical examination of the Medicaid rebate rules of 1990. Journal of Economics and Management Strategy 1997;6(1):151‐74.
Narine 1997 {published data only}
-
- Narine L, Senathirajah M. Pharmaceutical cost containment policies: intended and unintended impacts. Toronto (ON): University of Toronto; 1997; Report prepared for the Pharmaceutical Manufacturers Association of Canada. Toronto: Department of Health Administration, University of Toronto.
Narine 1999 {published data only}
Rikstrygdeverket {published data only}
-
- Rikstrygdeverket. Economic consequences of the reference price scheme [Norwegian] [Økonomiske konsekvenser av referanseprisordningen]. Oslo (Norway): Rikstrygdeverket; 1999; Rapport 4/99. 4/99.
Rothberg 2004 {published data only}
-
- Rothberg AD, Blignault J, Serfontein CB, Valodia B, Eekhout S, Pels LM. Experience of a medicines reference‐pricing model. South African Medical Journal 2004;94:183‐8. - PubMed
Schneeweiss 2004b {published data only}
-
- Schneeweiss S, Dormuth C, Grootendorst P, Soumerai S, Maclure M. Net health plan savings from reference pricing for angiotensin‐converting enzyme inhibitors in elderly British Columbia residents. Medical Care 2004;42(7):653‐60. - PubMed
Steyn 2007 {published data only}
-
- Steyn R, Burger JR, Serfontein JH, Lubbe MS. Influence of a new reference‐based pricing system in South Africa on the prevalence and cost of antidiabetic medicine: a pilot study [Influence of a new reference‐based pricing system in South Africa on the prevalence and cost of antidiabetic medicine: a pilot study]. International Journal of Pharmacy Practice 2007;15(4):307‐11.
Thomas 1998 {published data only}
-
- Thomas M, Mann J, Williams S. The impact of reference pricing on clinical lipid control. New Zealand Medical Journal 1998;August:292‐4. - PubMed
Tordoff 2008 {published data only}
-
- Tordoff JM, Norris PT, Reith DM. "Price management" and its impact on hospital pharmaceutical expenditure and the availability of medicines in New Zealand hospitals. Value in Health 2008;11(7):1214‐26. - PubMed
Ubeda 2007 {published data only}
-
- Ubeda A, Cardo E, Selles N, Broseta R, Trillo JL, Fernandez‐Llimos F. Antidepressant utilization in primary care in a Spanish region: impact of generic and reference‐based pricing policy (2000‐2004). Social Psychiatry and Psychiatric Epidemiology 2007;42(3):181‐8. - PubMed
Vieira 2006 {published data only}
-
- Vieira FS, Zucchi P. [Price differences between generic and innovator medicines in Brazil] [Diferencas de precos entre medicamentos genericos e de referencia no Brasil]. Revista de Saude Publica 2006;40(3):444‐9. - PubMed
References to studies awaiting assessment
Huang 2012 {published data only}
Lee 2006 {published and unpublished data}
-
- Lee YC, Yang MC, Huang YT, Liu CH, Chen SB. Impacts of cost containment strategies on pharmaceutical expenditures of the National Health Insurance in Taiwan, 1996–2003. Pharmacoeconomics 2006;24(9):891‐902. - PubMed
Additional references
Aaserud 2006a
-
- Aaserud M, Austvoll‐Dahlgren A, Sturm H, Kösters JP, Hill S, Furberg CD, et al. Pharmaceutical policies: effects on rational drug use (Protocol). Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004397.pub2] - DOI
Austvoll‐Dahlgren 2008
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Ciapponi 2011
-
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. ISPOR 3rd Latin America Conference. Hilton Mexico City Reforma in Mexico City, Mexico, 2011.
Danzon 2008
-
- Danzon PM, Furukawa MF. International prices and availability of pharmaceuticals in 2005. Health Affairs (Millwood) 2008;1(1):221‐33. - PubMed
Dickson 1998
-
- Dickson M, Redwood H. Pharmaceutical reference prices. How do they work in practice?. Pharmacoeconomics 1998;14(5):471‐9. - PubMed
EPOC 2013a
-
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2013b
-
- Effective Practice, Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC resources for review authors. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2013c
-
- Effective Practice, Organisation of Care (EPOC). Synthesising results when it does not make sense to do a meta‐analysis. EPOC Resources for review authors. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2013d
-
- Effective Practice, Organisation of Care (EPOC). EPOC Worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
Espin 2007
-
- Espin J, Rovira R. Analysis of differences and commonalities in pricing and reimbursement systems in Europe. European Commission 2007; Vol. http://ec.europa.eu/enterprise/sectors/healthcare/files/docs/study_prici....
Espin 2011
-
- Espin J, Rovira J, Olry de Labry A. Review Series on Pharmaceutical Pricing Policies and Interventions Working Paper 1: External Reference Pricing. Review Series on Pharmaceutical Pricing Policies and Interventions. WHO/HAI Project on Medicine Prices and Availability May 2011.
Faden 2011
-
- Faden L, Vialle‐Valentin C, Ross‐Degnan D, Wagner A. Active pharmaceutical management strategies of health insurance systems to improve cost‐effective use of medicines in low‐ and middle‐income countries: a systematic review of current evidence. Health Policy (Amsterdam, Netherlands) 2011;100(2‐3):134‐43. [PUBMED: 21185616] - PubMed
Festoy 2008
-
- Festöy H, Sveen K, Yu LM, Gjönnes L, Gregersen T. Pharmaceutical pricing and reimbursement information. Norwegian Medicines Agency 2008.
Galizzi 2011
-
- Galizzi MM, Ghislandi S, Miraldo M. Effects of reference pricing in pharmaceutical markets: a review. PharmacoEconomics 2011;29(1):17‐33. - PubMed
Glujovsky 2010
-
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. XVIII Cochrane Colloquium. The Joint Colloquium of the Cochrane and Campbell Collaborations. Keystone Resort, Colorado, USA, 2010.
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. [Computer program]. Version 3.2 for Windows. the GRADE Working Group, 2008.
Green 2010
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Ioannides‐Demos 2002
-
- Ioannides‐Demos LL, Ibrahim JE, McNeil JJ. Reference‐based pricing schemes: effect on pharmaceutical expenditure, resource utilisation and health outcomes. PharmacoEconomics 2002;20(9):577‐91. - PubMed
McLaughin 1997
-
- McLaughin P. Reference‐based pricing of prescription drugs. Canadian Journal of Cardiology 1997;13(1):31‐2. - PubMed
Mossialos 2004
-
- Mossialos E, Mrazek M, Walley T. Regulating pharmaceuticals in Europe: striving for efficiency, equity and quality. First Edition. Great Britain: Open University Press, McGraw‐Hill Education, 2004.
Moïse 2008
-
- Moïse P, Docteur E. Pharmaceuticals pricing and reimbursement policies in Mexico, OECD, 2007 [Las políticas de precios y reembolsos farmacéuticos en México, OCDE, 2007]. OECD Health Working Papers 2008;No. 29:s504‐10. - PubMed
Norwegian Pharmacy Association 2008
-
- The Norwegian Pharmacy Association. The medicines market in Norway ‐ prices and regulations. www.apotek.no August 2008.
OECD 2008
-
- OECD Health Data 2008 [Pharmaceutical Pricing Policies in a Global Market]. Organisation for Economic Co‐operation and Development. ORGANISATION FOR ECONOMIC CO‐OPERATION AND DEVELOPMENT, 2008.
Pharmanet 2003
-
- British Columbia Medical Association. Physician access to Pharmanet. http://www.bcma.org/public/news_publications/publications/policy_backgro... (accessed 18 January 2006).
Pharmanet 2005
-
- Ministry of Health in British Columbia. Pharmanet. http://www.healthservices.gov.bc.ca/pharme/pharmanet/netindex.html (accessed 18 January 2006).
PHIS 2011
-
- AIFA, Gesundheit Österreich GmbH. PHIS Glossary [Pharmaceutical Health Information System Glossary]. http://whocc.goeg.at/Literaturliste/Dokumente/MethodologyTemplate/PHIS%2... 2011:16, 25.
PPRS 2009
-
- Pharmaceutical Price Regulation Scheme, Tenth report Dec 2009. available at www.dh.gov.uk/publications [Accessed on 17th Oct 2011].
Productivity 2001
-
- Productivity Commission 2001. International pharmaceutical price differences. Canberra (AU): Research report, Ausinfo. Canberra.
Puig‐Junoy 2007
-
- Puig‐Junoy J, Moreno‐Torres I. Impact of pharmaceutical prior authorisation policies: a systematic review of the literature. PharmacoEconomics 2007;25(8):637‐48. [PUBMED: 17640106] - PubMed
Puig‐Junoy 2010
-
- Puig‐Junoy J. Impact of European pharmaceutical price regulation on generic price competition: a review. PharmacoEconomics 2010;28(8):649‐63. - PubMed
Puig‐Junoy 2010a
-
- Puig‐Junoy J. Policies encouraging price competition in the generic drug market: Lessons from the European experience [Polí́ticas de fomento de la competencia en precios en el mercado de genéricos: lecciones de la experiencia europea]. Gaceta Sanitaria 2010;24(3):193‐9. [MEDLINE: ] - PubMed
Ramsay 2003
-
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series design in health technology assessment: Lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):612‐23. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sturm 2007
Vacca 2011
-
- Vacca C, Acosta A, Rodriguez I. International reference prices and cost minimization analysis for pricing regulation in Colombia [Precios de Referencia Internacional y Análisis de Costo Minimización para la Regulación de Precios de Medicamentos en Colombia]. Value in Health July 2011;14(5):S16‐9. - PubMed
Vogler 2008
-
- Vogler S, Habl C, Leopold C, Rosian‐Schikuta I, Joncheere K, Thomsen T, et al. The Pharmaceutical Pricing and Reimbursement Information project Report [PPRI Report]. European Commission, Directorate‐General Health and Consumer Protection and Austrian Federal Ministry of Health, Family and Youth June, 2008:XVI‐XVIII.
WHO 2011
-
- The World Medicines Situation. WHO 2011.
Zammit‐Lucia 1995
-
- Zammit‐Lucia J, Dasgupta R. Reference pricing: the European experience. Health Policy Review. 10. England: St. Mary's Hospital Medical School, 1995; Vol. 10:1‐31.
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

