DNA supercoiling: a regulatory signal for the λ repressor
- PMID: 25319264
- PMCID: PMC4217475
- DOI: 10.1073/pnas.1320644111
DNA supercoiling: a regulatory signal for the λ repressor
Abstract
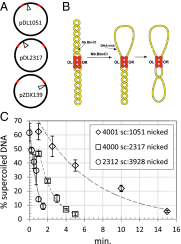
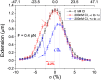
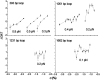
Topoisomerases, polymerases, and the chirality introduced by the binding of histones or nucleoid-associated proteins affect DNA supercoiling in vivo. However, supercoiling is not just a by-product of DNA metabolism. Supercoiling is an indicator of cell health, it modifies the accessibility of chromatin, and coordinates the transcription of genes. This suggests that regulatory, protein-mediated loops in DNA may sense supercoiling of the genome in which they are embedded. The λ repressor (CI) maintains the quiescent (lysogenic) transcriptome of bacteriophage λ in infected Escherichia coli. CI-mediated looping prevents overexpression of the repressor protein to preserve sensitivity to conditions that trigger virulence (lysis). Experiments were performed to assess how well the CI-mediated DNA loop traps superhelicity and determine whether supercoiling enhances CI-mediated DNA looping. CI oligomers partitioned plasmids into topological domains and prevented the passage of supercoiling between them. Furthermore, in single DNA molecules stretched and twisted with magnetic tweezers, levels of superhelical density confined in CI-mediated DNA loops ranged from -15% or +11%. Finally, in DNA under tensions that may occur in vivo, supercoiling lowered the free energy of loop formation and was essential for DNA looping. Supercoiling-enhanced looping can influence the maintenance of lysogeny in the λ repressor system; it can encode sensitivity to the energy level of the cell and creates independent topological domains of distinct superhelical density.
Keywords: DNA looping; DNA supercoiling; bacteriophage λ repressor; magnetic tweezer; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

