Effect of switching therapy to pegaptanib in eyes with the persistent cases of exudative age-related macular degeneration
- PMID: 25319441
- PMCID: PMC4616293
- DOI: 10.1097/MD.0000000000000116
Effect of switching therapy to pegaptanib in eyes with the persistent cases of exudative age-related macular degeneration
Abstract
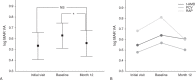
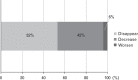
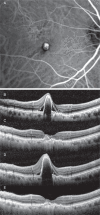
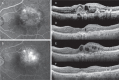
Purpose of this study was to evaluate the efficacy of switching to pegaptanib monotherapy for persistent cases of exudative age-related macular degeneration (AMD).Out of 296 eyes of 296 patients treated with ranibizumab or ranibizumab combined with photodynamic therapy (PDT), 50 eyes of 50 AMD patients were found to be resistant to these treatments. Over a 12-month period, intravitreal pegaptanib (IVP) 0.3 mg was administered at intervals of 6 weeks until the exudation disappeared prospectively. All patients were examined with the following tests: best-corrected visual acuity (BCVA) and central retinal thickness (CRT), determined at the initial visit, before the first IVP (baseline), and at 12 months. The factors responsible for achieving dry macula with IVP were examined statistically.The rate of persistent cases with intravitreal ranibizumab (IVR) and/or PDT was 17.0%. The mean number of IVPs administered was 5.4 (range, 2-9). Logarithm of the minimal angle of resolution BCVA at 12 months was stable or improved by ≥ 0.3 in 49 eyes (98.0%), with a significant improvement noted between the baseline and final BCVA (P=0.01, paired t test). The CRT (mean ± standard deviation) was 446.9 ± 150.6 µm at the initial visit, 414.5 ± 146.5 µm at baseline, and 318.7 ± 99.0 µm at 12 months. There was a significant decrease in the mean CRT between the measurements at baseline and at 12 months after the first IVP (P=0.002, Bonferroni correction). At 12 months, the exudative change was completely resolved in 27 eyes (54.0%) and reduced in 21 eyes (42.0%). The number of previous IVR treatments was significantly correlated with dry macula at 12 months.After switching therapy to pegaptanib in persistent cases of AMD, most patients maintained or improved their BCVA and exhibited a positive treatment response at 12 months.
Conflict of interest statement
The authors have no funding to disclose.
The authors have no proprietary interest in any aspect of this study.
Figures

References
-
- Apte RS, Modi M, Masonson H, et al. ; Macugen AMD Study Group. Pegaptanib 1-year systemic safety results from a safety-pharmacokinetic trial in patients with neovascular age-related macular degeneration. Ophthalmology. 2007;114:1702–1712. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Boyer DS, Antoszyk AN, Awh CC, et al. ; MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

