Insights into the renal pathogenesis in Schimke immuno-osseous dysplasia: A renal histological characterization and expression analysis
- PMID: 25319549
- PMCID: PMC4395996
- DOI: 10.1369/0022155414558335
Insights into the renal pathogenesis in Schimke immuno-osseous dysplasia: A renal histological characterization and expression analysis
Abstract
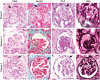
Schimke immuno-osseous dysplasia (SIOD) is a pleiotropic disorder caused by mutations in the SWI/SNF2-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like-1 (SMARCAL1) gene, with multiple clinical features, notably end-stage renal disease. Here we characterize the renal pathology in SIOD patients. Our analysis of SIOD patient renal biopsies demonstrates the tip and collapsing variants of focal segmental glomerulosclerosis (FSGS). Additionally, electron microscopy revealed numerous glomerular abnormalities most notably in the podocyte and Bowman's capsule. To better understand the role of SMARCAL1 in the pathogenesis of FSGS, we defined SMARCAL1 expression in the developing and mature kidney. In the developing fetal kidney, SMARCAL1 is expressed in the ureteric epithelium, stroma, metanephric mesenchyme, and in all stages of the developing nephron, including the maturing glomerulus. In postnatal kidneys, SMARCAL1 expression is localized to epithelial tubules of the nephron, collecting ducts, and glomerulus (podocytes and endothelial cells). Interestingly, not all cells within the same lineage expressed SMARCAL1. In renal biopsies from SIOD patients, TUNEL analysis detected marked increases in DNA fragmentation. Our results highlight the cells that may contribute to the renal pathogenesis in SIOD. Further, we suggest that disruptions in genomic integrity during fetal kidney development contribute to the pathogenesis of FSGS in SIOD patients.
Keywords: DNA fragmentation; FSGS; SIOD; SMARCAL1; Schimke immuno-osseous dysplasia; focal segmental glomerulosclerosis; kidney; kidney pathology.
© The Author(s) 2014.
Conflict of interest statement
Figures





References
-
- Boerkoel CF, O’Neill S, Andre JL, Benke PJ, Bogdanovic R, Bulla M, Burguet A, Cockfield S, Cordeiro I, Ehrich JH, Frund S, Geary DF, Ieshima A, Illies F, Joseph MW, Kaitila I, Lama G, Leheup B, Ludman MD, McLeod DR, Medeira A, Milford DV, Ormala T, Rener-Primec Z, Santava A, Santos HG, Schmidt B, Smith GC, Spranger J, Zupancic N, Weksberg R. (2000). Manifestations and treatment of Schimke immuno-osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr 159:1-7. - PubMed
-
- Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, Andre JL, Bogdanovic R, Burguet A, Cockfield S, Cordeiro I, Frund S, Illies F, Joseph M, Kaitila I, Lama G, Loirat C, McLeod DR, Milford DV, Petty EM, Rodrigo F, Saraiva JM, Schmidt B, Smith GC, Spranger J, Stein A, Thiele H, Tizard J, Weksberg R, Lupski JR, Stockton DW. (2002). Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet 30:215-220. - PubMed
-
- Choi MJ. (2013). Histologic classification of FSGS: does form delineate function? Clin J Am Soc Nephrol 8:344-346. - PubMed
-
- Cohen A. (2006). Renal Anatomy and Basic Concepts and Methods in Renal pathology. In Agnes FJ Charles., Brujin Jan., Colvin Robert., (eds.) Fundamentals of Renal Pathology. New York, NY: Springer, pp3-19.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

