LRP4 is critical for neuromuscular junction maintenance
- PMID: 25319686
- PMCID: PMC4198535
- DOI: 10.1523/JNEUROSCI.1733-14.2014
LRP4 is critical for neuromuscular junction maintenance
Erratum in
- J Neurosci. 2015 May 13;35(19):7655
Abstract
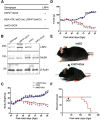
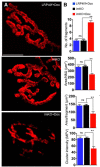
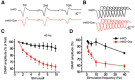
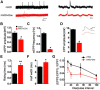
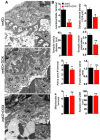
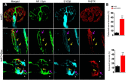
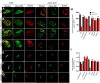
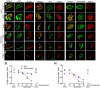
The neuromuscular junction (NMJ) is a synapse between motor neurons and skeletal muscle fibers, and is critical for control of muscle contraction. Its formation requires neuronal agrin that acts by binding to LRP4 to stimulate MuSK. Mutations have been identified in agrin, MuSK, and LRP4 in patients with congenital myasthenic syndrome, and patients with myasthenia gravis develop antibodies against agrin, LRP4, and MuSK. However, it remains unclear whether the agrin signaling pathway is critical for NMJ maintenance because null mutation of any of the three genes is perinatal lethal. In this study, we generated imKO mice, a mutant strain whose LRP4 gene can be deleted in muscles by doxycycline (Dox) treatment. Ablation of the LRP4 gene in adult muscle enabled studies of its role in NMJ maintenance. We demonstrate that Dox treatment of P30 mice reduced muscle strength and compound muscle action potentials. AChR clusters became fragmented with diminished junctional folds and synaptic vesicles. The amplitude and frequency of miniature endplate potentials were reduced, indicating impaired neuromuscular transmission and providing cellular mechanisms of adult LRP4 deficiency. We showed that LRP4 ablation led to the loss of synaptic agrin and the 90 kDa fragments, which occurred ahead of other prejunctional and postjunctional components, suggesting that LRP4 may regulate the stability of synaptic agrin. These observations demonstrate that LRP4 is essential for maintaining the structural and functional integrity of the NMJ and that loss of muscle LRP4 in adulthood alone is sufficient to cause myasthenic symptoms.
Keywords: AChRs; LRP4; NMJ; agrin; congenital myasthenic syndrome; synaptic basal lamina.
Copyright © 2014 the authors 0270-6474/14/3413892-14$15.00/0.
Figures

References
-
- Barik A, Xiong Wc, Mei L. Mukai H, editor. MuSK: a kinase critical for the formation and maintenance of the neuromuscular junction. Protein kinase technologies. 2012;vol. 68:203–217.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
