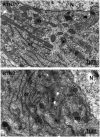
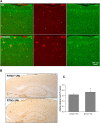
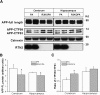
Impact of RTN3 deficiency on expression of BACE1 and amyloid deposition
- PMID: 25319692
- PMCID: PMC4198539
- DOI: 10.1523/JNEUROSCI.1588-14.2014
Impact of RTN3 deficiency on expression of BACE1 and amyloid deposition
Abstract
Reticulon 3 (RTN3) has previously been shown to interact with BACE1 and negatively regulate BACE1 activity. To what extent RTN3 deficiency affects BACE1 activity is an intriguing question. In this study, we aimed to address this by generating RTN3-null mice. Mice with complete deficiency of RTN3 grow normally and have no obviously discernible phenotypes. Morphological analyses of RTN3-null mice showed no significant alterations in cellular structure, although RTN3 is recognized as a protein contributing to the shaping of tubular endoplasmic reticulum. Biochemical analysis revealed that RTN3 deficiency increased protein levels of BACE1. This elevation of BACE1 levels correlated with enhanced processing of amyloid precursor protein at the β-secretase site. We also demonstrated that RTN3 deficiency in Alzheimer's mouse models facilitates amyloid deposition, further supporting an in vivo role of RTN3 in the regulation of BACE1 activity. Since it has been shown that RTN3 monomer is reduced in brains of Alzheimer's patients, our results suggest that long-lasting reduction of RTN3 levels has adverse effects on BACE1 activity and may contribute to Alzheimer's pathogenesis.
Keywords: APP; Alzheimer's disease; BACE1; Nogo; neuritic plaques; reticulon.
Copyright © 2014 the authors 0270-6474/14/3413954-09$15.00/0.
Figures



Similar articles
-
Effects of altered RTN3 expression on BACE1 activity and Alzheimer's neuritic plaques.Rev Neurosci. 2017 Feb 1;28(2):145-154. doi: 10.1515/revneuro-2016-0054. Rev Neurosci. 2017. PMID: 27883331 Review.
-
RTN1 and RTN3 protein are differentially associated with senile plaques in Alzheimer's brains.Sci Rep. 2017 Jul 21;7(1):6145. doi: 10.1038/s41598-017-05504-9. Sci Rep. 2017. PMID: 28733667 Free PMC article.
-
Reduction of β-amyloid accumulation by reticulon 3 in transgenic mice.Curr Alzheimer Res. 2013 Feb;10(2):135-42. doi: 10.2174/1567205011310020003. Curr Alzheimer Res. 2013. PMID: 22742855
-
Increased expression of reticulon 3 in neurons leads to reduced axonal transport of β site amyloid precursor protein-cleaving enzyme 1.J Biol Chem. 2013 Oct 18;288(42):30236-30245. doi: 10.1074/jbc.M113.480079. Epub 2013 Sep 4. J Biol Chem. 2013. PMID: 24005676 Free PMC article.
-
Regulation of β-site APP-cleaving enzyme 1 gene expression and its role in Alzheimer's disease.J Neurochem. 2012 Jan;120 Suppl 1:62-70. doi: 10.1111/j.1471-4159.2011.07515.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 22122349 Review.
Cited by
-
Reticulon 3 regulates sphingosine-1-phosphate synthesis in endothelial cells to control blood pressure.MedComm (2020). 2024 Feb 13;5(2):e480. doi: 10.1002/mco2.480. eCollection 2024 Feb. MedComm (2020). 2024. PMID: 38352050 Free PMC article.
-
Identification of rare RTN3 variants in Alzheimer's disease in Han Chinese.Hum Genet. 2018 Feb;137(2):141-150. doi: 10.1007/s00439-018-1868-1. Epub 2018 Jan 22. Hum Genet. 2018. PMID: 29356939
-
ER-phagy: shaping up and destressing the endoplasmic reticulum.FEBS J. 2019 Jul;286(14):2645-2663. doi: 10.1111/febs.14932. Epub 2019 Jun 10. FEBS J. 2019. PMID: 31116513 Free PMC article. Review.
-
Disruption of BAG3-mediated BACE1 stabilization alleviates neuropathology and memory deficits in a mouse model of Alzheimer's disease.Sci Adv. 2025 May 23;11(21):eadt7981. doi: 10.1126/sciadv.adt7981. Epub 2025 May 23. Sci Adv. 2025. PMID: 40408490 Free PMC article.
-
Function of Nogo-A/Nogo-A receptor in Alzheimer's disease.CNS Neurosci Ther. 2015 Jun;21(6):479-85. doi: 10.1111/cns.12387. Epub 2015 Mar 2. CNS Neurosci Ther. 2015. PMID: 25732725 Free PMC article. Review.
References
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/S0896-6273(00)80974-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
