Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer
- PMID: 25320012
- PMCID: PMC4498250
- DOI: 10.1158/0008-5472.CAN-14-1913
Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer
Abstract
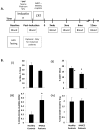
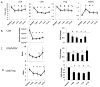
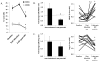
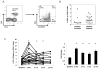
While viral antigens in human papillomavirus (HPV)-related oropharyngeal cancer (HPVOPC) are attractive targets for immunotherapy, the effects of existing standard-of-care therapies on immune responses to HPV are poorly understood. We serially sampled blood from patients with stage III-IV oropharyngeal cancer undergoing concomitant chemoradiotherapy with or without induction chemotherapy. Circulating immunocytes including CD4(+) and CD8(+) T cells, regulatory T cells (Treg), and myeloid-derived suppressor cells (MDSC) were profiled by flow cytometry. Antigen-specific T-cell responses were measured in response to HPV16 E6 and E7 peptide pools. The role of PD-1 signaling in treatment-related immunosuppression was functionally defined by performing HPV-specific T-cell assays in the presence of blocking antibody. While HPV-specific T-cell responses were present in 13 of 18 patients before treatment, 10 of 13 patients lost these responses within 3 months after chemoradiotherapy. Chemoradiotherapy decreased circulating T cells and markedly elevated MDSCs. PD-1 expression on CD4(+) T cells increased by nearly 2.5-fold after chemoradiotherapy, and ex vivo culture with PD-1-blocking antibody enhanced HPV-specific T-cell responses in 8 of 18 samples tested. Chemoradiotherapy suppresses circulating immune responses in patients with HPVOPC by unfavorably altering effector:suppressor immunocyte ratios and upregulating PD-1 expression on CD4(+) T cells. These data strongly support testing of PD-1-blocking agents in combination with standard-of-care chemoradiotherapy for HPVOPC.
©2014 American Association for Cancer Research.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURE STATEMENT:
The corresponding author is the recipient of an unrestricted industry grant from Advaxis Pharmaceuticals for an investigator-initiated immunotherapy trial for HPV-positive oropharyngeal cancer.
Figures


References
-
- Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer J Int Cancer. 2009;125:362–6. - PubMed
-
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. - PubMed
-
- Münger K, Scheffner M, Huibregtse JM, Howley PM. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

