The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo
- PMID: 25320048
- PMCID: PMC4279099
- DOI: 10.1124/jpet.114.216820
The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo
Abstract
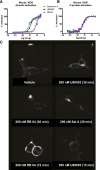
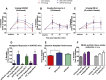
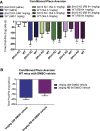
The hypothesis that functionally selective G protein-coupled receptor (GPCR) agonists may have enhanced therapeutic benefits has revitalized interest for many GPCR targets. In particular, although κ-opioid receptor (KOR) agonists are analgesic with a low risk of dependence and abuse, their use is limited by a propensity to induce sedation, motor incoordination, hallucinations, and dysphoria-like states. Several laboratories have produced a body of work suggesting that G protein-biased KOR agonists might be analgesic with fewer side effects. Although that has been an intriguing hypothesis, suitable KOR-selective and G protein-biased agonists have not been available to test this idea. Here we provide data using a G protein-biased agonist, RB-64 (22-thiocyanatosalvinorin A), which suggests that KOR-mediated G protein signaling induces analgesia and aversion, whereas β-arrestin-2 signaling may be associated with motor incoordination. Additionally, unlike unbiased KOR agonists, the G protein-biased ligand RB-64 does not induce sedation and does not have anhedonia-like actions, suggesting that a mechanism other than G protein signaling mediates these effects. Our findings provide the first evidence for a highly selective and G protein-biased tool compound for which many, but not all, of the negative side effects of KOR agonists can be minimized by creating G protein-biased KOR agonists.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Allen JA, Roth BL. (2011) Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 51:117–144. - PubMed
-
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, et al. (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther 318:641–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

