Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo
- PMID: 25320076
- PMCID: PMC4256360
- DOI: 10.1074/jbc.M114.601104
Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo
Abstract
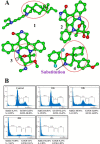
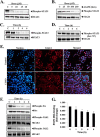
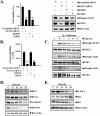
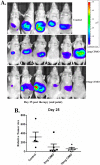
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that regulates genes involved in cell growth, proliferation, and survival, and given its association with many types of cancers, it has recently emerged as a promising target for therapy. In this work, we present the synthesis of N-substituted azaspirane derivatives and their biological evaluation against hepatocellular carcinoma (HCC) cells (IC50 = 7.3 μm), thereby identifying 2-(1-(4-(2-cyanophenyl)1-benzyl-1H-indol-3-yl)-5-(4-methoxy-phenyl)-1-oxa-3-azaspiro(5,5) undecane (CIMO) as a potent inhibitor of the JAK-STAT pathway with selectivity over normal LO2 cells (IC50 > 100 μm). The lead compound, CIMO, suppresses proliferation of HCC cells and achieves this effect by reducing both constitutive and inducible phosphorylation of JAK1, JAK2, and STAT3. Interestingly, CIMO displayed inhibition of Tyr-705 phosphorylation, which is required for nuclear translocation of STAT3, but it has no effect on Ser-727 phosphorylation. CIMO accumulates cancer cells in the sub-G1 phase and decreases STAT3 in the nucleus and thereby causes down-regulation of genes regulated via STAT3. Suppression of STAT3 phosphorylation by CIMO and knockdown of STAT3 mRNA using siRNA transfection displayed a similar effect on the viability of HCC cells. Furthermore, CIMO significantly decreased the tumor development in an orthotopic HCC mouse model through the modulation of phospho-STAT3, Ki-67, and cleaved caspase-3 in tumor tissues. Thus, CIMO represents a chemically novel and biologically in vitro and in vivo validated compound, which targets the JAK-STAT pathway as a potential cancer treatment.
Keywords: Bioinformatics; Cell Migration; Hepatocellular Carcinoma; Janus Kinase (JAK); STAT3.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
An azaspirane derivative suppresses growth and induces apoptosis of ER-positive and ER-negative breast cancer cells through the modulation of JAK2/STAT3 signaling pathway.Int J Oncol. 2016 Sep;49(3):1221-9. doi: 10.3892/ijo.2016.3615. Epub 2016 Jul 6. Int J Oncol. 2016. PMID: 27500741
-
Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3.Br J Pharmacol. 2013 Oct;170(4):807-21. doi: 10.1111/bph.12302. Br J Pharmacol. 2013. PMID: 23848338 Free PMC article.
-
A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway.Oncotarget. 2015 Oct 13;6(31):31927-43. doi: 10.18632/oncotarget.5578. Oncotarget. 2015. PMID: 26376676 Free PMC article.
-
Synthesis, biological activities and mechanistic studies of C20-ketone pachysandra alkaloids as anti-hepatocellular carcinoma agents.Mol Divers. 2025 Jun;29(3):2617-2637. doi: 10.1007/s11030-024-10961-2. Epub 2024 Aug 19. Mol Divers. 2025. PMID: 39158620 Review.
-
Deciphering STAT3 signaling potential in hepatocellular carcinoma: tumorigenesis, treatment resistance, and pharmacological significance.Cell Mol Biol Lett. 2023 Apr 21;28(1):33. doi: 10.1186/s11658-023-00438-9. Cell Mol Biol Lett. 2023. PMID: 37085753 Free PMC article. Review.
Cited by
-
A brief overview of antitumoral actions of bruceine D.Explor Target Antitumor Ther. 2020;1(4):200-217. doi: 10.37349/etat.2020.00013. Epub 2020 Aug 31. Explor Target Antitumor Ther. 2020. PMID: 36046775 Free PMC article. Review.
-
Omega-3 Polyunsaturated Fatty Acids Provoke Apoptosis in Hepatocellular Carcinoma through Knocking Down the STAT3 Activated Signaling Pathway: In Vivo and In Vitro Study.Molecules. 2022 May 9;27(9):3032. doi: 10.3390/molecules27093032. Molecules. 2022. PMID: 35566382 Free PMC article.
-
Targeting Cancer Stem Cells by Dietary Agents: An Important Therapeutic Strategy against Human Malignancies.Int J Mol Sci. 2021 Oct 28;22(21):11669. doi: 10.3390/ijms222111669. Int J Mol Sci. 2021. PMID: 34769099 Free PMC article. Review.
-
Endophytic Fungi-Alternative Sources of Cytotoxic Compounds: A Review.Front Pharmacol. 2018 Apr 26;9:309. doi: 10.3389/fphar.2018.00309. eCollection 2018. Front Pharmacol. 2018. PMID: 29755344 Free PMC article. Review.
-
Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance.Int J Mol Sci. 2020 Jun 3;21(11):4002. doi: 10.3390/ijms21114002. Int J Mol Sci. 2020. PMID: 32503307 Free PMC article. Review.
References
-
- Whang-Peng J., Cheng A.-L., Hsu C., Chen C.-M. (2010) Clinical development and future direction for the treatment of hepatocellular carcinoma. J. Exp. Clin. Med. 2, 93–103
-
- Rajendran P., Ong T. H., Chen L., Li F., Shanmugam M. K., Vali S., Abbasi T., Kapoor S., Sharma A., Kumar A. P., Hui K. M., Sethi G. (2011) Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 17, 1425–1439 - PubMed
-
- Wörns M. A., Galle P. R. (2010) Future perspectives in hepatocellular carcinoma. Dig. Liver Dis. 42, S302–S309 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

