A novel class of somatic small RNAs similar to germ cell pachytene PIWI-interacting small RNAs
- PMID: 25320077
- PMCID: PMC4239631
- DOI: 10.1074/jbc.M114.613232
A novel class of somatic small RNAs similar to germ cell pachytene PIWI-interacting small RNAs
Abstract
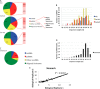
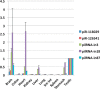
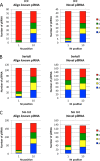
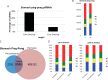
PIWI-interacting RNAs (piRNAs) are small noncoding RNAs that bind PIWI family proteins exclusively expressed in the germ cells of mammalian gonads. MIWI2-associated piRNAs are essential for silencing transposons during primordial germ cell development, and MIWI-bound piRNAs are required for normal spermatogenesis during adulthood in mice. Although piRNAs have long been regarded as germ cell-specific, increasing lines of evidence suggest that somatic cells also express piRNA-like RNAs (pilRNAs). Here, we report the detection of abundant pilRNAs in somatic cells, which are similar to MIWI-associated piRNAs mainly expressed in pachytene spermatocytes and round spermatids in the testis. Based on small RNA deep sequencing and quantitative PCR analyses, pilRNA expression is dynamic and displays tissue specificity. Although pilRNAs are similar to pachytene piRNAs in both size and genomic origins, they have a distinct ping-pong signature. Furthermore, pilRNA biogenesis appears to utilize a yet to be identified pathway, which is different from all currently known small RNA biogenetic pathways. In addition, pilRNAs appear to preferentially target the 3'-UTRs of mRNAs in a partially complementary manner. Our data suggest that pilRNAs, as an integral component of the small RNA transcriptome in somatic cell lineages, represent a distinct population of small RNAs that may have functions similar to germ cell piRNAs.
Keywords: Gene Regulation; RNA; RNA Interference (RNAi); Somatic Cell Genetics; Transposable Element (TE).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Heat stress induced piRNA alterations in pachytene spermatocytes and round spermatids.Reprod Biol Endocrinol. 2024 Jul 24;22(1):87. doi: 10.1186/s12958-024-01249-z. Reprod Biol Endocrinol. 2024. PMID: 39049033 Free PMC article.
-
yama, a mutant allele of Mov10l1, disrupts retrotransposon silencing and piRNA biogenesis.PLoS Genet. 2021 Feb 26;17(2):e1009265. doi: 10.1371/journal.pgen.1009265. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33635934 Free PMC article.
-
Bovine piRNA-like RNAs are associated with both transposable elements and mRNAs.Reproduction. 2017 Mar;153(3):305-318. doi: 10.1530/REP-16-0620. Epub 2016 Dec 13. Reproduction. 2017. PMID: 27965401
-
Enigmatic Pachytene PIWI-Interacting RNAs.Genome Biol Evol. 2024 Oct 9;16(10):evae162. doi: 10.1093/gbe/evae162. Genome Biol Evol. 2024. PMID: 39056586 Free PMC article. Review.
-
The functions and mechanisms of piRNAs in mediating mammalian spermatogenesis and their applications in reproductive medicine.Cell Mol Life Sci. 2024 Sep 2;81(1):379. doi: 10.1007/s00018-024-05399-6. Cell Mol Life Sci. 2024. PMID: 39222270 Free PMC article. Review.
Cited by
-
Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm.Epigenetics Chromatin. 2021 May 24;14(1):24. doi: 10.1186/s13072-021-00397-5. Epigenetics Chromatin. 2021. PMID: 34030709 Free PMC article.
-
Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology.Sci Rep. 2015 May 27;5:10423. doi: 10.1038/srep10423. Sci Rep. 2015. PMID: 26013764 Free PMC article.
-
Non-coding RNAs regulate mitochondrial dynamics in the development of gastric cancer.Front Mol Biosci. 2023 Jan 12;10:1107651. doi: 10.3389/fmolb.2023.1107651. eCollection 2023. Front Mol Biosci. 2023. PMID: 36714260 Free PMC article. Review.
-
The roles of non-coding RNAs in cardiac regenerative medicine.Noncoding RNA Res. 2017 Jun 7;2(2):100-110. doi: 10.1016/j.ncrna.2017.06.001. eCollection 2017 Jun. Noncoding RNA Res. 2017. PMID: 30159427 Free PMC article. Review.
-
Somatic expression of piRNA and associated machinery in the mouse identifies short, tissue-specific piRNA.Epigenetics. 2019 May;14(5):504-521. doi: 10.1080/15592294.2019.1600389. Epub 2019 Apr 8. Epigenetics. 2019. PMID: 30955436 Free PMC article.
References
-
- Aravin A., Gaidatzis D., Pfeffer S., Lagos-Quintana M., Landgraf P., Iovino N., Morris P., Brownstein M. J., Kuramochi-Miyagawa S., Nakano T., Chien M., Russo J. J., Ju J., Sheridan R., Sander C., Zavolan M., Tuschl T. (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 - PubMed
-
- Girard A., Sachidanandam R., Hannon G. J., Carmell M. A. (2006) A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202 - PubMed
-
- Lau N. C., Seto A. G., Kim J., Kuramochi-Miyagawa S., Nakano T., Bartel D. P., Kingston R. E. (2006) Characterization of the piRNA complex from rat testes. Science 313, 363–367 - PubMed
-
- Watanabe T., Takeda A., Tsukiyama T., Mise K., Okuno T., Sasaki H., Minami N., Imai H. (2006) Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 20, 1732–1743 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

