Assembly and function of the major histocompatibility complex (MHC) I peptide-loading complex are conserved across higher vertebrates
- PMID: 25320083
- PMCID: PMC4246071
- DOI: 10.1074/jbc.M114.609263
Assembly and function of the major histocompatibility complex (MHC) I peptide-loading complex are conserved across higher vertebrates
Abstract
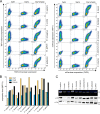
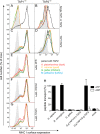
Antigen presentation to cytotoxic T lymphocytes via major histocompatibility complex class I (MHC I) molecules depends on the heterodimeric transporter associated with antigen processing (TAP). For efficient antigen supply to MHC I molecules in the ER, TAP assembles a macromolecular peptide-loading complex (PLC) by recruiting tapasin. In evolution, TAP appeared together with effector cells of adaptive immunity at the transition from jawless to jawed vertebrates and diversified further within the jawed vertebrates. Here, we compared TAP function and interaction with tapasin of a range of species within two classes of jawed vertebrates. We found that avian and mammalian TAP1 and TAP2 form heterodimeric complexes across taxa. Moreover, the extra N-terminal domain TMD0 of mammalian TAP1 and TAP2 as well as avian TAP2 recruits tapasin. Strikingly, however, only TAP1 and TAP2 from the same taxon can form a functional heterodimeric translocation complex. These data demonstrate that the dimerization interface between TAP1 and TAP2 and the tapasin docking sites for PLC assembly are conserved in evolution, whereas elements of antigen translocation diverged later in evolution and are thus taxon specific.
Keywords: ABC Transporter; Antigen Processing; Immunology; Major Histocompatibility Complex (MHC); Membrane Protein; Transporter.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Identification of domain boundaries within the N-termini of TAP1 and TAP2 and their importance in tapasin binding and tapasin-mediated increase in peptide loading of MHC class I.Immunol Cell Biol. 2005 Oct;83(5):475-82. doi: 10.1111/j.1440-1711.2005.01354.x. Immunol Cell Biol. 2005. PMID: 16174096
-
Peptide-bound major histocompatibility complex class I molecules associate with tapasin before dissociation from transporter associated with antigen processing.J Biol Chem. 1999 Mar 26;274(13):8649-54. doi: 10.1074/jbc.274.13.8649. J Biol Chem. 1999. PMID: 10085102
-
Three tapasin docking sites in TAP cooperate to facilitate transporter stabilization and heterodimerization.J Immunol. 2014 Mar 1;192(5):2480-94. doi: 10.4049/jimmunol.1302637. Epub 2014 Feb 5. J Immunol. 2014. PMID: 24501197 Free PMC article.
-
What is the role of alternate splicing in antigen presentation by major histocompatibility complex class I molecules?Immunol Res. 2010 Mar;46(1-3):32-44. doi: 10.1007/s12026-009-8123-8. Immunol Res. 2010. PMID: 19830395 Free PMC article. Review.
-
The transporter associated with antigen processing: a key player in adaptive immunity.Biol Chem. 2015 Sep;396(9-10):1059-72. doi: 10.1515/hsz-2014-0320. Biol Chem. 2015. PMID: 25781678 Review.
Cited by
-
Dual role of the peptide-loading complex as proofreader and limiter of MHC-I presentation.Proc Natl Acad Sci U S A. 2024 May 28;121(22):e2321600121. doi: 10.1073/pnas.2321600121. Epub 2024 May 21. Proc Natl Acad Sci U S A. 2024. PMID: 38771881 Free PMC article.
-
Structure of the transporter associated with antigen processing trapped by herpes simplex virus.Elife. 2016 Dec 9;5:e21829. doi: 10.7554/eLife.21829. Elife. 2016. PMID: 27935481 Free PMC article.
-
A dual inhibition mechanism of herpesviral ICP47 arresting a conformationally thermostable TAP complex.Sci Rep. 2016 Nov 15;6:36907. doi: 10.1038/srep36907. Sci Rep. 2016. PMID: 27845362 Free PMC article.
-
Genome-wide SNP identification for the construction of a high-resolution genetic map of Japanese flounder (Paralichthys olivaceus): applications to QTL mapping of Vibrio anguillarum disease resistance and comparative genomic analysis.DNA Res. 2015 Apr;22(2):161-70. doi: 10.1093/dnares/dsv001. Epub 2015 Mar 11. DNA Res. 2015. PMID: 25762582 Free PMC article.
-
Relationship between HLA-DPA1 genetic polymorphism and anembryonic pregnancy.Mol Genet Genomic Med. 2020 Jan;8(1):e1046. doi: 10.1002/mgg3.1046. Epub 2019 Nov 30. Mol Genet Genomic Med. 2020. PMID: 31785132 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

