Oligomerization, membrane association, and in vivo phosphorylation of sugarcane UDP-glucose pyrophosphorylase
- PMID: 25320091
- PMCID: PMC4246093
- DOI: 10.1074/jbc.M114.590125
Oligomerization, membrane association, and in vivo phosphorylation of sugarcane UDP-glucose pyrophosphorylase
Abstract
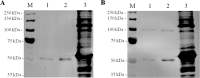
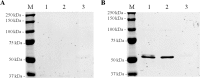
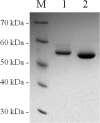
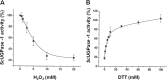
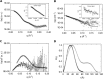
Sugarcane is a monocot plant that accumulates sucrose to levels of up to 50% of dry weight in the stalk. The mechanisms that are involved in sucrose accumulation in sugarcane are not well understood, and little is known with regard to factors that control the extent of sucrose storage in the stalks. UDP-glucose pyrophosphorylase (UGPase; EC 2.7.7.9) is an enzyme that produces UDP-glucose, a key precursor for sucrose metabolism and cell wall biosynthesis. The objective of this work was to gain insights into the ScUGPase-1 expression pattern and regulatory mechanisms that control protein activity. ScUGPase-1 expression was negatively correlated with the sucrose content in the internodes during development, and only slight differences in the expression patterns were observed between two cultivars that differ in sucrose content. The intracellular localization of ScUGPase-1 indicated partial membrane association of this soluble protein in both the leaves and internodes. Using a phospho-specific antibody, we observed that ScUGPase-1 was phosphorylated in vivo at the Ser-419 site in the soluble and membrane fractions from the leaves but not from the internodes. The purified recombinant enzyme was kinetically characterized in the direction of UDP-glucose formation, and the enzyme activity was affected by redox modification. Preincubation with H2O2 strongly inhibited this activity, which could be reversed by DTT. Small angle x-ray scattering analysis indicated that the dimer interface is located at the C terminus and provided the first structural model of the dimer of sugarcane UGPase in solution.
Keywords: Gene Expression; Kinetics; Protein Phosphorylation; Redox Regulation; Small Angle X-ray Scattering (SAXS); Sucrose.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




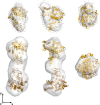
References
-
- Waclawovsky A. J., Sato P. M., Lembke C. G., Moore P. H., Souza G. M. (2010) Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 8, 263–276 - PubMed
-
- Matsuoka S., Ferro J., Arruda P. (2009) The Brazilian experience of sugarcane ethanol industry. Vitr. Cell. Dev. Biol. Plant 45, 372–381
-
- Arruda P. (2012) Genetically modified sugarcane for bioenergy generation. Curr. Opin. Biotechnol. 23, 315–322 - PubMed
-
- Botha F. C., Black K. G. (2000) Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Funct. Plant Biol. 27, 81–85
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

