The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease
- PMID: 25320178
- PMCID: PMC4203382
- DOI: 10.1158/0008-5472.CAN-14-1531
The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease
Abstract
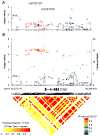
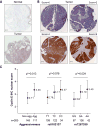
A genome-wide association study (GWAS) of bladder cancer identified a genetic marker rs8102137 within the 19q12 region as a novel susceptibility variant. This marker is located upstream of the CCNE1 gene, which encodes cyclin E, a cell-cycle protein. We performed genetic fine-mapping analysis of the CCNE1 region using data from two bladder cancer GWAS (5,942 cases and 10,857 controls). We found that the original GWAS marker rs8102137 represents a group of 47 linked SNPs (with r(2) ≥ 0.7) associated with increased bladder cancer risk. From this group, we selected a functional promoter variant rs7257330, which showed strong allele-specific binding of nuclear proteins in several cell lines. In both GWASs, rs7257330 was associated only with aggressive bladder cancer, with a combined per-allele OR = 1.18 [95% confidence interval (CI), 1.09-1.27, P = 4.67 × 10(-5)] versus OR = 1.01 (95% CI, 0.93-1.10, P = 0.79) for nonaggressive disease, with P = 0.0015 for case-only analysis. Cyclin E protein expression analyzed in 265 bladder tumors was increased in aggressive tumors (P = 0.013) and, independently, with each rs7257330-A risk allele (P(trend) = 0.024). Overexpression of recombinant cyclin E in cell lines caused significant acceleration of cell cycle. In conclusion, we defined the 19q12 signal as the first GWAS signal specific for aggressive bladder cancer. Molecular mechanisms of this genetic association may be related to cyclin E overexpression and alteration of cell cycle in carriers of CCNE1 risk variants. In combination with established bladder cancer risk factors and other somatic and germline genetic markers, the CCNE1 variants could be useful for inclusion into bladder cancer risk prediction models.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures

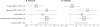

References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. - PubMed
-
- Hernandez S, Lopez-Knowles E, Lloreta J, Kogevinas M, Amoros A, Tardon A, Carrato A, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–3671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01HG004446/HG/NHGRI NIH HHS/United States
- CA34627/CA/NCI NIH HHS/United States
- UM1 CA176726/CA/NCI NIH HHS/United States
- HHSN268201100046C/HL/NHLBI NIH HHS/United States
- DH_/Department of Health/United Kingdom
- R01CA65726/CA/NCI NIH HHS/United States
- R01 CA082838/CA/NCI NIH HHS/United States
- R01CA114665/CA/NCI NIH HHS/United States
- P01 CA87969/CA/NCI NIH HHS/United States
- HHSN268200782096C/HL/NHLBI NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- N01 CN045165/CN/NCI NIH HHS/United States
- 1R01CA114665/CA/NCI NIH HHS/United States
- CA082838/CA/NCI NIH HHS/United States
- R01 CA057494/CA/NCI NIH HHS/United States
- 16491/CRUK_/Cancer Research UK/United Kingdom
- U01HG004438/HG/NHGRI NIH HHS/United States
- N01-RC-45035/RC/CCR NIH HHS/United States
- P50 CA091846/CA/NCI NIH HHS/United States
- U01 HG004438/HG/NHGRI NIH HHS/United States
- T32 GM007814/GM/NIGMS NIH HHS/United States
- U01 HG004446/HG/NHGRI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- R01 CA67262/CA/NCI NIH HHS/United States
- R01 CA067262/CA/NCI NIH HHS/United States
- HHSN261201000006C/CP/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- BHF_/British Heart Foundation/United Kingdom
- R01 CA065726/CA/NCI NIH HHS/United States
- N02 CP001037/CP/NCI NIH HHS/United States
- N02 CP011015/CP/NCI NIH HHS/United States
- P01 CA055075/CA/NCI NIH HHS/United States
- R01-CA089715/CA/NCI NIH HHS/United States
- HHSN268201100003C/WH/WHI NIH HHS/United States
- R01 CA089715/CA/NCI NIH HHS/United States
- R01 CA49449/CA/NCI NIH HHS/United States
- R01 CA114665/CA/NCI NIH HHS/United States
- N01 CN045035/CN/NCI NIH HHS/United States
- 1P01CA86871/CA/NCI NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
- HHSN271201100004C/AG/NIA NIH HHS/United States
- HHSN268201100002C/WH/WHI NIH HHS/United States
- R01HL091172-01/HL/NHLBI NIH HHS/United States
- P01 CA086871/CA/NCI NIH HHS/United States
- R01 CA034627/CA/NCI NIH HHS/United States
- UM1 CA167552/CA/NCI NIH HHS/United States
- N01 RC037004/RC/CCR NIH HHS/United States
- N02-CP-01037/CP/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 CA049449/CA/NCI NIH HHS/United States
- CA055075/CA/NCI NIH HHS/United States
- 001/WHO_/World Health Organization/International
- HHSN268201100001C/WH/WHI NIH HHS/United States
- HHSN268201100004C/WH/WHI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

