SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer
- PMID: 25320180
- PMCID: PMC4203414
- DOI: 10.1158/0008-5472.CAN-14-1308
SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer
Abstract
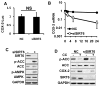
SIRT6 is a SIR2 family member that regulates multiple molecular pathways involved in metabolism, genomic stability, and aging. It has been proposed previously that SIRT6 is a tumor suppressor in cancer. Here, we challenge this concept by presenting evidence that skin-specific deletion of SIRT6 in the mouse inhibits skin tumorigenesis. SIRT6 promoted expression of COX-2 by repressing AMPK signaling, thereby increasing cell proliferation and survival in the skin epidermis. SIRT6 expression in skin keratinocytes was increased by exposure to UVB light through activation of the AKT pathway. Clinically, we found that SIRT6 was upregulated in human skin squamous cell carcinoma. Taken together, our results provide evidence that SIRT6 functions as an oncogene in the epidermis and suggest greater complexity to its role in epithelial carcinogenesis.
©2014 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest.
Figures





References
-
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. - PubMed
-
- Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-Blight signalling. Nat Rev Cancer. 2004;4:23–35. - PubMed
-
- Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat Rev Cancer. 2005;5:564–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

