Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia
- PMID: 25320232
- PMCID: PMC4476552
- DOI: 10.1126/scitranslmed.3010157
Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia
Abstract
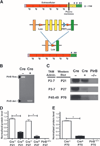
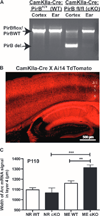
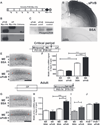
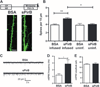
During critical periods of development, the brain easily changes in response to environmental stimuli, but this neural plasticity declines by adulthood. By acutely disrupting paired immunoglobulin-like receptor B (PirB) function at specific ages, we show that PirB actively represses neural plasticity throughout life. We disrupted PirB function either by genetically introducing a conditional PirB allele into mice or by minipump infusion of a soluble PirB ectodomain (sPirB) into mouse visual cortex. We found that neural plasticity, as measured by depriving mice of vision in one eye and testing ocular dominance, was enhanced by this treatment both during the critical period and when PirB function was disrupted in adulthood. Acute blockade of PirB triggered the formation of new functional synapses, as indicated by increases in miniature excitatory postsynaptic current (mEPSC) frequency and spine density on dendrites of layer 5 pyramidal neurons. In addition, recovery from amblyopia--the decline in visual acuity and spine density resulting from long-term monocular deprivation--was possible after a 1-week infusion of sPirB after the deprivation period. Thus, neural plasticity in adult visual cortex is actively repressed and can be enhanced by blocking PirB function.
Copyright © 2014, American Association for the Advancement of Science.
Figures

References
-
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16:1412–1425. - PubMed
-
- Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu. Rev. Neurosci. 2012;35:309–330. - PubMed
-
- Levi DM. Visual processing in amblyopia: Human studies. Strabismus. 2006;14:11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

