An antiapoptotic role for telomerase RNA in human immune cells independent of telomere integrity or telomerase enzymatic activity
- PMID: 25320237
- PMCID: PMC4263978
- DOI: 10.1182/blood-2014-06-582254
An antiapoptotic role for telomerase RNA in human immune cells independent of telomere integrity or telomerase enzymatic activity
Abstract
Telomerase is a ribonucleoprotein complex that adds telomeric DNA to the ends of linear chromosomes. It contains two core canonical components: the essential RNA component, hTR, which provides the template for DNA synthesis, and the reverse transcriptase protein component, hTERT. Low telomerase activity in circulating peripheral blood mononuclear cells has been associated with a variety of diseases. It is unknown, however, whether telomerase, in addition to its long-term requirement for telomere maintenance, is also necessary for short-term immune cell proliferation and survival. We report that overexpression of enzymatically inactive hTR mutants protected against dexamethasone-induced apoptosis in stimulated CD4 T cells. Furthermore, hTR knockdown reproducibly induced apoptosis in the absence of any detectable telomere shortening or DNA damage response. In contrast, hTERT knockdown did not induce apoptosis. Strikingly, overexpression of hTERT protein caused apoptosis that was rescued by overexpression of enzymatically inactive hTR mutants. Hence, we propose that hTR can function as a noncoding RNA that protects from apoptosis independent of its function in telomerase enzymatic activity and long-term telomere maintenance in normal human immune cells. These results imply that genetic or environmental factors that alter hTR levels can directly affect immune cell function to influence health and disease.
© 2014 by The American Society of Hematology.
Figures
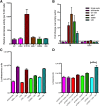

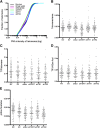
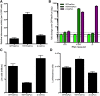

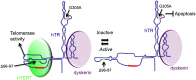
References
-
- Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. - PubMed
-
- Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

