Regulatory T cells in central nervous system injury: a double-edged sword
- PMID: 25320276
- PMCID: PMC4225170
- DOI: 10.4049/jimmunol.1302401
Regulatory T cells in central nervous system injury: a double-edged sword
Abstract
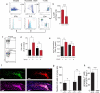
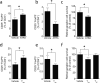
Previous research investigating the roles of T effector (T(eff)) and T regulatory (T(reg)) cells after injury to the CNS has yielded contradictory conclusions, with both protective and destructive functions being ascribed to each of these T cell subpopulations. In this work, we study this dichotomy by examining how regulation of the immune system affects the response to CNS trauma. We show that, in response to CNS injury, T(eff) and T(reg) subsets in the CNS-draining deep cervical lymph nodes are activated, and surgical resection of these lymph nodes results in impaired neuronal survival. Depletion of T(reg), not surprisingly, induces a robust T(eff) response in the draining lymph nodes and is associated with impaired neuronal survival. Interestingly, however, injection of exogenous T(reg) cells, which limits the spontaneous beneficial immune response after CNS injury, also impairs neuronal survival. We found that no T(reg) accumulate at the site of CNS injury, and that changes in T(reg) numbers do not alter the amount of infiltration by other immune cells into the site of injury. The phenotype of macrophages at the site, however, is affected: both addition and removal of T(reg) negatively impact the numbers of macrophages with alternatively activated (tissue-building) phenotype. Our data demonstrate that neuronal survival after CNS injury is impaired when T(reg) cells are either removed or added. With this exacerbation of neurodegeneration seen with both addition and depletion of T(reg), we recommend exercising extreme caution when considering the therapeutic targeting of T(reg) cells after CNS injury, and possibly in chronic neurodegenerative conditions.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures




References
-
- Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Experimental Neuroloy. 1998;153:1–7. - PubMed
-
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. - PubMed
-
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

