A 2.5-kilobase deletion containing a cluster of nine microRNAs in the latency-associated-transcript locus of the pseudorabies virus affects the host response of porcine trigeminal ganglia during established latency
- PMID: 25320324
- PMCID: PMC4301172
- DOI: 10.1128/JVI.02181-14
A 2.5-kilobase deletion containing a cluster of nine microRNAs in the latency-associated-transcript locus of the pseudorabies virus affects the host response of porcine trigeminal ganglia during established latency
Abstract
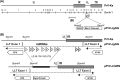
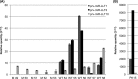
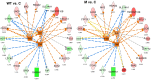
The alphaherpesvirus pseudorabies virus (PrV) establishes latency primarily in neurons of trigeminal ganglia when only the transcription of the latency-associated transcript (LAT) locus is detected. Eleven microRNAs (miRNAs) cluster within the LAT, suggesting a role in establishment and/or maintenance of latency. We generated a mutant (M) PrV deleted of nine miRNA genes which displayed properties that were almost identical to those of the parental PrV wild type (WT) during propagation in vitro. Fifteen pigs were experimentally infected with either WT or M virus or were mock infected. Similar levels of virus excretion and host antibody response were observed in all infected animals. At 62 days postinfection, trigeminal ganglia were excised and profiled by deep sequencing and quantitative RT-PCR. Latency was established in all infected animals without evidence of viral reactivation, demonstrating that miRNAs are not essential for this process. Lower levels of the large latency transcript (LLT) were found in ganglia infected by M PrV than in those infected by WT PrV. All PrV miRNAs were expressed, with highest expression observed for prv-miR-LLT1, prv-miR-LLT2 (in WT ganglia), and prv-miR-LLT10 (in both WT and M ganglia). No evidence of differentially expressed porcine miRNAs was found. Fifty-four porcine genes were differentially expressed between WT, M, and control ganglia. Both viruses triggered a strong host immune response, but in M ganglia gene upregulation was prevalent. Pathway analyses indicated that several biofunctions, including those related to cell-mediated immune response and the migration of dendritic cells, were impaired in M ganglia. These findings are consistent with a function of the LAT locus in the modulation of host response for maintaining a latent state.
Importance: This study provides a thorough reference on the establishment of latency by PrV in its natural host, the pig. Our results corroborate the evidence obtained from the study of several LAT mutants of other alphaherpesviruses encoding miRNAs from their LAT regions. Neither PrV miRNA expression nor high LLT expression levels are essential to achieve latency in trigeminal ganglia. Once latency is established by PrV, the only remarkable differences are found in the pattern of host response. This indicates that, as in herpes simplex virus, LAT functions as an immune evasion locus.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Typical gene expression profile of pseudorabies virus reactivation from latency in swine trigeminal ganglion.J Neurovirol. 2020 Oct;26(5):687-695. doi: 10.1007/s13365-020-00866-9. Epub 2020 Jul 15. J Neurovirol. 2020. PMID: 32671812
-
Pseudorabies virus infected porcine epithelial cell line generates a diverse set of host microRNAs and a special cluster of viral microRNAs.PLoS One. 2012;7(1):e30988. doi: 10.1371/journal.pone.0030988. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292087 Free PMC article.
-
Potential sites of virus latency associated with indigenous pseudorabies viruses in feral swine.J Wildl Dis. 2003 Jul;39(3):567-75. doi: 10.7589/0090-3558-39.3.567. J Wildl Dis. 2003. PMID: 14567217
-
A Comparison of Pseudorabies Virus Latency to Other α-Herpesvirinae Subfamily Members.Viruses. 2022 Jun 24;14(7):1386. doi: 10.3390/v14071386. Viruses. 2022. PMID: 35891367 Free PMC article. Review.
-
The Role of Latency-Associated Transcripts in the Latent Infection of Pseudorabies Virus.Viruses. 2022 Jun 24;14(7):1379. doi: 10.3390/v14071379. Viruses. 2022. PMID: 35891360 Free PMC article. Review.
Cited by
-
Emerging Roles of Herpesvirus microRNAs During In Vivo Infection and Pathogenesis.Curr Pathobiol Rep. 2015;3(3):209-217. doi: 10.1007/s40139-015-0085-z. Curr Pathobiol Rep. 2015. PMID: 26246961 Free PMC article. Review.
-
Molecular Aspects of Varicella-Zoster Virus Latency.Viruses. 2018 Jun 28;10(7):349. doi: 10.3390/v10070349. Viruses. 2018. PMID: 29958408 Free PMC article. Review.
-
Roles of Non-coding RNAs During Herpesvirus Infection.Curr Top Microbiol Immunol. 2018;419:243-280. doi: 10.1007/82_2017_31. Curr Top Microbiol Immunol. 2018. PMID: 28674945 Free PMC article. Review.
-
A previously unidentified circRNA inhibits virus replication by regulating the miR-24-3p/KEAP1 axis.PLoS Pathog. 2024 Dec 17;20(12):e1012712. doi: 10.1371/journal.ppat.1012712. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39689152 Free PMC article.
-
Computational Network Analysis Identifies Evolutionarily Conserved miRNA Gene Interactions Potentially Regulating Immune Response in Bovine Trypanosomosis.Front Microbiol. 2019 Aug 28;10:2010. doi: 10.3389/fmicb.2019.02010. eCollection 2019. Front Microbiol. 2019. PMID: 31555241 Free PMC article.
References
-
- Enquist LW. 1994. Infection of the mammalian nervous system by pseudorabies virus (PRV). Semin Virol 5:221–231. doi:10.1006/smvy.1994.1024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

