Adjuvant therapy for gastric cancer: current and future directions
- PMID: 25320509
- PMCID: PMC4194555
- DOI: 10.3748/wjg.v20.i38.13718
Adjuvant therapy for gastric cancer: current and future directions
Abstract
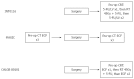
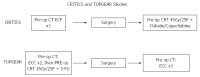
The management of gastric cancer continues to evolve. Whilst surgery alone is effective when tumours present early, a large proportion of patients are diagnosed with loco-regionally advanced disease, resulting in high loco-regional and distant relapse rates, with subsequent poor survival. Early attempts at improving outcomes following resection were disappointing; however, randomized trials have now established either post-operative chemoradiotherapy (INT0116) or peri-operative chemotherapy as standard adjuvant therapies in the Western world. There remain, however, significant differences in the approach to management between the West and East. In Asia, where there is the highest incidence of gastric cancer, extended resection followed by adjuvant chemotherapy represents the standard of care. This review discusses current standard adjuvant therapy in gastric adenocarcinoma, as well as recent and ongoing trials investigating novel (neo)adjuvant approaches, which hope to build on the successes of previous studies.
Keywords: Adjuvant; Cancer; Chemoradiation; Chemoradiotherapy; Chemotherapy; Gastric; Neo-adjuvant; Peri-operative; Stomach.
Figures
References
-
- Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon: Int. Agency Res. Cancer; 2010.
-
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer; 2010.
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. - PubMed
-
- Greene FL, Page D, Fleming I. Fritz A, Balch CM, Haller DG, Morrow M. American Joint Committee on Cancer Staging Manual. 6th ed. New York: Springer; 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

