Gastric cancer: prevention, screening and early diagnosis
- PMID: 25320521
- PMCID: PMC4194567
- DOI: 10.3748/wjg.v20.i38.13842
Gastric cancer: prevention, screening and early diagnosis
Abstract
Gastric cancer continues to be an important healthcare problem from a global perspective. Most of the cases in the Western world are diagnosed at late stages when the treatment is largely ineffective. Helicobacter pylori (H. pylori) infection is a well-established carcinogen for gastric cancer. While lifestyle factors are important, the efficacy of interventions in their modification, as in the use of antioxidant supplements, is unconvincing. No organized screening programs can be found outside Asia (Japan and South Korea). Although several screening approaches have been proposed, including indirect atrophy detection by measuring pepsinogen in the circulation, none of them have so far been implemented, and more study data is required to justify any implementation. Mass eradication of H. pylori in high-risk areas tends to be cost-effective, but its adverse effects and resistance remain a concern. Searches for new screening biomarkers, including microRNA and cancer-autoantibody panels, as well as detection of volatile organic compounds in the breath, are in progress. Endoscopy with a proper biopsy follow-up remains the standard for early detection of cancer and related premalignant lesions. At the same time, new advanced high-resolution endoscopic technologies are showing promising results with respect to diagnosing mucosal lesions visually and targeting each biopsy. New histological risk stratifications (classifications), including OLGA and OLGIM, have recently been developed. This review addresses the current means for gastric cancer primary and secondary prevention, the available and emerging methods for screening, and new developments in endoscopic detection of early lesions of the stomach.
Keywords: Gastric cancer; Helicobacter pylori.
Figures
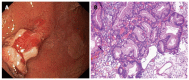
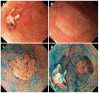
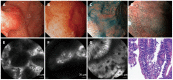
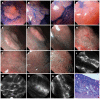
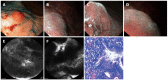
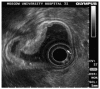
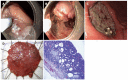
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. - PubMed
-
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. - PubMed
-
- González CA, Megraud F, Buissonniere A, Lujan Barroso L, Agudo A, Duell EJ, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, Krogh V, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23:1320–1324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

