Combination and switching of stimulants in children and adolescents with attention deficit/hyperactivity disorder in quebec
- PMID: 25320609
- PMCID: PMC4197516
Combination and switching of stimulants in children and adolescents with attention deficit/hyperactivity disorder in quebec
Abstract
Objective: To assess the one-year period prevalence of stimulant combination therapy and switching in children/ adolescents with attention deficit/hyperactivity disorder (ADHD) in Quebec, Canada.
Method: Patients aged 6-17 years, with at least two ADHD diagnosis codes documented in different visits and at least 30 days' supply of a stimulant during their most recent one-year observation period were selected from the Regie de l'assurance maladie du Quebec database (03/2007-02/2012). Combination therapy was defined as at least 30 consecutive days of concomitant use of multiple stimulants with different active moieties, or use of a stimulant and another psychotropic medication. Therapy switching was defined as a prescription claim for a new psychotropic medication less than 30 days before or after the end of supply of a stimulant. The one-year period prevalence of therapy combination and switching was calculated.
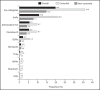
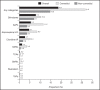
Results: The one-year period prevalence of combination therapy and switching among 9,431 children and adolescents with ADHD treated with stimulants was 19.8% and 18.7%, respectively. The most frequent combination categories were atypical antipsychotics (AAP: 10.8%), atomoxetine (ATX: 5.5%) and clonidine (5.3%). The most frequent switched-to categories were other stimulants (7.9%), AAP (5.5%) and ATX (4.7%).
Conclusions: Approximately one in five children/adolescents with ADHD on a stimulant experienced combination therapy or therapy switching; however, the majority of the medications used in combination or switching were not label-indicated for the treatment of ADHD in Canada. These results highlight the need for further research to evaluate the risk-benefit of stimulant combination and switching in children and adolescents with ADHD.
Objectif: Évaluer la prévalence sur une période d’un an de la traitement par combinaison et par changement de stimulants chez les enfants et les adolescents souffrant du trouble de déficit de l’attention avec hyperactivité (TDAH) au Québec, Canada.
Méthode: Des patients de 6 à 17 ans, ayant au moins deux codes diagnostiques de TDAH documentés à différentes visites et une provision d’au moins 30 jours d’un stimulant durant leur plus récente période d’observation d’un an, ont été choisis dans la base de données de la Régie de l’assurance maladie du Québec (03/2007–02/2012). La traitement par combinaison a été définie comme étant au moins 30 jours consécutifs d’utilisation concomitante de multiples stimulants ayant différentes parties actives, ou d’utilisation d’un stimulant et d’un autre médicament psychotrope. La traitement par changement a été définie comme étant une demande de prescription d’un nouveau médicament psychotrope moins de 30 jours avant ou après la fin d’une provision d’un stimulant. La prévalence sur une période d’un an de la traitement par combinaison et par changement a été calculée.
Résultats: La prévalence sur une période d’un an de la traitement par combinaison et par changement chez 9 431 enfants et adolescents souffrant de TDAH traités par stimulants était de 19,8% et 18,7%, respectivement. Les catégories de combinaison les plus fréquentes étaient les antipsychotiques atypiques (APA: 10,8%), l’atomoxétine (ATX: 5,5%) et la clonidine (5,3%). Les catégories pour lesquelles les changements se faisaient le plus souvent étaient d’autres stimulants (7,9%), les APA (5,5%) et l’ATX (4,7%).
Conclusions: Environ un enfant/adolescent sur cinq qui souffrent de TDAH et prennent des stimulants ont fait l’expérience d’une thérapie par combinaison ou par changement; toutefois, la majorité des médicaments utilisés en combinaison ou pour le changement n’étaient pas indiqués sur l’étiquette pour le traitement du TDAH au Canada. Ces résultats font ressortir le besoin de plus de recherche pour évaluer les risques-avantages de la combinaison et du changement de stimulants chez les enfants et adolescents souffrant de TDAH.
Keywords: ADHD; RAMQ; combination therapy; stimulants; switching.
Figures

References
-
- Alessi-Severini S, Biscontri RG, Collins DM, Sareen J, Enns MW. Ten years of antipsychotic prescribing to children: A Canadian population-based study. Canadian Journal of Psychiatry Revue canadienne de psychiatrie. 2012;57(1):52–58. - PubMed
-
- Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: A placebo-controlled pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(5):558–565. - PubMed
-
- Balkrishnan R, Pollack M, Joish VN, Asche CV, Pawaskar MD, Cziraky MJ. An economic evaluation of therapeutic alteration in the management of insomnia. Current Medical Research and Opinion. 2009;25(3):663–669. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
