Topical corticosteroids as adjunctive therapy for bacterial keratitis
- PMID: 25321340
- PMCID: PMC4269217
- DOI: 10.1002/14651858.CD005430.pub3
Topical corticosteroids as adjunctive therapy for bacterial keratitis
Abstract
Background: Bacterial keratitis is a serious ocular infectious disease that can lead to severe visual disability. Risk factors for bacterial corneal infection include contact lens wear, ocular surface disease, corneal trauma, and previous ocular or eyelid surgery. Topical antibiotics constitute the mainstay of treatment in cases of bacterial keratitis, whereas the use of topical corticosteroids as an adjunctive therapy to antibiotics remains controversial. Topical corticosteroids are usually used to control inflammation using the smallest amount of the drug. Their use requires optimal timing, concomitant antibiotics, and careful follow-up.
Objectives: The objective of the review was to assess the effectiveness and safety of corticosteroids as adjunctive therapy for bacterial keratitis. Secondary objectives included evaluation of health economic outcomes and quality of life outcomes.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2014), EMBASE (January 1980 to July 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to July 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 July 2014. We also searched the Science Citation Index to identify additional studies that had cited the only trial included in the original version of this review, reference lists of included trials, earlier reviews, and the American Academy of Ophthalmology guidelines. We also contacted experts to identify any unpublished and ongoing randomized trials.
Selection criteria: We included randomized controlled trials (RCTs) that had evaluated adjunctive therapy with topical corticosteroids in people with bacterial keratitis who were being treated with antibiotics.
Data collection and analysis: We used the standard methodological procedures expected by The Cochrane Collaboration.
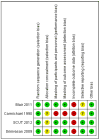
Main results: We found four RCTs that met the inclusion criteria of this review. The total number of included participants was 611 (612 eyes), ranging from 30 to 500 participants per trial. One trial was included in the previous version of the review, and we identified three additional trials through the updated searches in July 2014. One of the three smaller trials was a pilot study of the largest study: the Steroids for Corneal Ulcers Trial (SCUT). All trials compared the treatment of bacterial keratitis with topical corticosteroid and without topical corticosteroid and had follow-up periods ranging from two months to one year. These trials were conducted in the USA, Canada, India, and South Africa.All trials reported data on visual acuity ranging from three weeks to one year, and none of them found any important difference between the corticosteroid group and the control group. The pilot study of the SCUT reported that time to re-epithelialization in the steroid group was 53% slower than the placebo group after adjusting for baseline epithelial defect size (hazard ratio (HR) 0.47; 95% confidence interval (CI) 0.23 to 0.94). However, the SCUT did not find any important difference in time to re-epithelialization (HR 0.92; 95% CI 0.76 to 1.11). For adverse events, none of the three small trials found any important difference between the two treatment groups. The investigators of the largest trial reported that more patients in the control group developed intraocular pressure (IOP) elevation (risk ratio (RR) 0.20; 95% CI 0.04 to 0.90). One trial reported quality of life and concluded that there was no difference between the two groups (data not available). We did not find any reports regarding economic outcomes.Although the four trials were generally of good methodological design, all trials had considerable losses to follow-up (10% or more) in the final analyses. Further, three of the four trials were underpowered to detect treatment effect differences between groups and inconsistency in outcome measurements precluded meta-analyses for most outcomes relevant to this review.
Authors' conclusions: There is inadequate evidence as to the effectiveness and safety of adjunctive topical corticosteroids compared with no topical corticosteroids in improving visual acuity, infiltrate/scar size, or adverse events among participants with bacterial keratitis. Current evidence does not support a strong effect of corticosteroid, but may be due to insufficient power to detect a treatment effect.
Conflict of interest statement
Xue Wang: none known
Johann MG Reyes: none known
Figures


Update of
-
Topical corticosteroids as adjunctive therapy for bacterial keratitis.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD005430. doi: 10.1002/14651858.CD005430.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2014 Oct 16;(10):CD005430. doi: 10.1002/14651858.CD005430.pub3. PMID: 17943856 Free PMC article. Updated.
References
References to studies included in this review
-
- Blair J, Hodge W, Al-Ghamdi S, Balabanian R, Lowcock B, Pan YI, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: double-blinded randomized clinical trial. Canada Journal of Ophthalmology. 2011;46(1):40–5. - PubMed
-
- Hodge WG, Blair J, Al-Ghambdi S, Balabanian R, Lowcock BC, Pan YI, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: a double-blinded, randomized clinical trial - interim report. Investigative Ophthalmology and Visual Science. 2007;48 ARVO E-Abstract 4275. - PubMed
-
- Acharya N, Srinivasan M, Mahalakshmi R, Jayashree D, Prajna L, House J, et al. Steroids for Corneal Ulcers Treatment - SCUT pilot study results. Investigative Ophthalmology and Visual Science. 2006;47 ARVO Eabstract 4752.
-
- Chen A, Prajna L, Srinivasan M, Acharya N, Lietman T. Does in vitro susceptibility testing predict clinical outcomes in bacterial keratitis? Results from the Steroids for Corneal Ulcers Trial (SCUT) pilot study. Investigative Ophthalmology and Visual Science. 2007;48 ARVO E-Abstract 4277.
References to studies excluded from this review
-
- Barequet IS, Jabbur NS, Barron Y, Osterhout GJ, O’Brien TP. Perioperative microbiologic profile of the conjunctiva in photorefractive keratectomy. Journal of Refractive Surgery. 2001;17(1):55–62. - PubMed
-
- Buhren J, Baumeister M, Kohnen T. Diffuse lamellar keratitis after laser in situ keratomileusis imaged by confocal microscopy. Ophthalmology. 2001;108(6):1075–81. - PubMed
-
- Callegan MC, O’Callaghan RJ, Hill JM. Pharmacokinetic considerations in the treatment of bacterial keratitis. Clinical Pharmacokinetics. 1994;27(2):129–49. - PubMed
-
- Cosar CB, Sener AB, Sen N, Coskunseven E. The efficacy of hourly prophylactic steroids in diffuse lamellar keratitis epidemic. Ophthalmologica. 2004;218(5):318–22. - PubMed
-
- Dighiero PL, Merciã M, Gicquel J. Early use of amniotic membrane transplantation combined with topical steroids in severe bacterial keratitis. Investigative Ophthalmology and Visual Science. 2005;45 ARVO E-abstract 2767.
References to ongoing studies
-
- NCT01721694. Association of azithromycin 1,5% loteprednol 0,5%eye drops versus individual administration of azithromycin 1,5%and loteprednol 0,5%in the treatment of ocular inflammation and infection. [3 December 2013]; clinicaltrials.gov/show/NCT01721694.
Additional references
-
- American Academy of Ophthalmology. American Academy of Ophthalmology Final Program (conference abstracts), Chicago. San Francisco, USA: American Academy of Ophthalmology; 2003.
-
- Bacterial keratitis Preferred Practice Pattern (PPP) guideline. American Academy of Ophthalmology. 2013
-
- Baker RS, Flowers CW, Jr, Casey R, Fong DS, Wilson MR. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Archives of Ophthalmology. 1996;114(5):632–3. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

