Enrichment of c-Met+ tumorigenic stromal cells of giant cell tumor of bone and targeting by cabozantinib
- PMID: 25321478
- PMCID: PMC4237261
- DOI: 10.1038/cddis.2014.440
Enrichment of c-Met+ tumorigenic stromal cells of giant cell tumor of bone and targeting by cabozantinib
Abstract
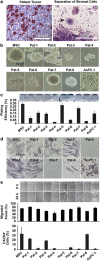
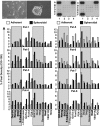
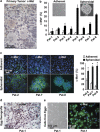
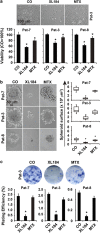
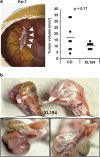
Giant cell tumor of bone (GCTB) is a very rare tumor entity, which is little examined owing to the lack of established cell lines and mouse models and the restriction of available primary cell lines. The stromal cells of GCTB have been made responsible for the aggressive growth and metastasis, emphasizing the presence of a cancer stem cell population. To identify and target such tumor-initiating cells, stromal cells were isolated from eight freshly resected GCTB tissues. Tumorigenic properties were examined by colony and spheroid formation, differentiation, migration, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, immunohistochemistry, antibody protein array, Alu in situ hybridization, FACS analysis and xenotransplantation into fertilized chicken eggs and mice. A sub-population of the neoplastic stromal cells formed spheroids and colonies, differentiated to osteoblasts, migrated to wounded regions and expressed the metastasis marker CXC-chemokine receptor type 4, indicating self-renewal, invasion and differentiation potential. Compared with adherent-growing cells, markers for pluripotency, stemness and cancer progression, including the CSC surface marker c-Met, were enhanced in spheroidal cells. This c-Met-enriched sub-population formed xenograft tumors in fertilized chicken eggs and mice. Cabozantinib, an inhibitor of c-Met in phase II trials, eliminated CSC features with a higher therapeutic effect than standard chemotherapy. This study identifies a c-Met(+) tumorigenic sub-population within stromal GCTB cells and suggests the c-Met inhibitor cabozantinib as a new therapeutic option for targeted elimination of unresectable or recurrent GCTB.
Figures


References
-
- 1Wulling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol 2001; 127: 467–474. - PubMed
-
- 2Zheng MH, Robbins P, Xu J, Huang L, Wood DJ, Papadimitriou JM. The histogenesis of giant cell tumour of bone: a model of interaction between neoplastic cells and osteoclasts. Histol Histopathol 2001; 16: 297–307. - PubMed
-
- 3Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol 2006; 29: 96–99. - PubMed
-
- 4Alberghini M, Kliskey K, Krenacs T, Picci P, Kindblom L, Forsyth R et al. Morphological and immunophenotypic features of primary and metastatic giant cell tumour of bone. Virchows Arch 2010; 456: 97–103. - PubMed
-
- 5Szendroi M. Giant-cell tumour of bone. J Bone Joint Surg Br 2004; 86: 5–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

