Placebo response in antipsychotic clinical trials: a meta-analysis
- PMID: 25321611
- PMCID: PMC4256120
- DOI: 10.1001/jamapsychiatry.2014.1319
Placebo response in antipsychotic clinical trials: a meta-analysis
Erratum in
- JAMA Psychiatry. 2015 Jan;72(1):96
Abstract
Importance: Because increasing placebo response rates decrease drug-placebo differences and increase the number of failed trials, it is imperative to determine what is causing this trend.
Objectives: To investigate the relationship between antipsychotic medication and placebo response by publication year, and to identify associated study design and implementation variables.
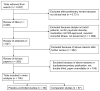
Data sources: MEDLINE, PsycINFO, and PubMed were searched to identify randomized clinical trials of antipsychotic medications published from 1960 to July 2013.
Study selection: Included were randomized clinical trials lasting 4 to 24 weeks, contrasting antipsychotic medication with placebo or an active comparator, and enrolling patients 18 years of age or older with schizophrenia or schizoaffective disorder.
Data extraction and synthesis: Standardized mean change scores were calculated for each treatment arm, plotted against publication year, and tested with Spearman rank correlation coefficients. Hierarchical linear modeling identified factors associated with the standardized mean change across medication and placebo treatment arms.
Main outcomes and measures: We hypothesized that the mean change in placebo-treated patients would significantly increase from 1960 to the present, that a greater change would be observed in active comparator vs placebo-controlled trials, and that more protocol visits would increase the symptom change observed.
Results: In the 105 trials examined, the mean change observed in placebo arms increased significantly with year of publication (n=39, r=0.52, P=.001), while the mean change in effective dose medication arms decreased significantly (n=208, r=-0.26, P<.001). Significant interactions were found between assignment to effective dose medication and publication year (t260=-5.55, P<.001), baseline severity (t260=5.08, P<.001), and study duration (t260=-3.76, P<.001), indicating that the average drug-placebo difference significantly decreased over time, with decreasing baseline severity and with increasing study duration. Medication treatment in comparator studies was associated with significantly more improvement than medication treatment in placebo-controlled trials (t93=2.73, P=.008).
Conclusions and relevance: The average treatment change associated with placebo treatment in antipsychotic trials increased since 1960, while the change associated with medication treatment decreased. Changes in randomized clinical trials leading to inflation of baseline scores, enrollment of less severely ill participants, and higher expectations of patients may all be responsible.
Figures

Comment in
-
Measuring the effects of treatment with antipsychotics.JAMA Psychiatry. 2015 May;72(5):513-4. doi: 10.1001/jamapsychiatry.2014.3136. JAMA Psychiatry. 2015. PMID: 25945489 No abstract available.
-
Measuring the effects of treatment with antipsychotics.JAMA Psychiatry. 2015 May;72(5):514. doi: 10.1001/jamapsychiatry.2014.3130. JAMA Psychiatry. 2015. PMID: 25945490 No abstract available.
-
Measuring the effects of treatment with antipsychotics.JAMA Psychiatry. 2015 May;72(5):514-5. doi: 10.1001/jamapsychiatry.2014.3134. JAMA Psychiatry. 2015. PMID: 25945491 No abstract available.
-
Measuring the effects of treatment with antipsychotics--reply.JAMA Psychiatry. 2015 May;72(5):515-6. doi: 10.1001/jamapsychiatry.2014.3184. JAMA Psychiatry. 2015. PMID: 25945492 No abstract available.
-
SSRI antidepressants are not associated with cerebral microbleeds or ischaemic vascular lesions.Evid Based Ment Health. 2015 Aug;18(3):82. doi: 10.1136/eb-2014-101972. Epub 2015 Jun 25. Evid Based Ment Health. 2015. PMID: 26112320 Free PMC article. No abstract available.
-
Increasing placebo response in antipsychotic trials: a clinical perspective.Evid Based Ment Health. 2015 Aug;18(3):77-9. doi: 10.1136/eb-2015-102098. Epub 2015 Jun 29. Evid Based Ment Health. 2015. PMID: 26124311 Free PMC article.
References
-
- Kinon BJ, Potts AJ, Watson SB. Placebo response in clinical trials with schizophrenia patients. Curr Opin Psychiatry. 2011;24:107–113. - PubMed
-
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo Response in Studies of Major Depression: Variable, Substantial, and Growing. JAMA. 2002;287:1840–1847. - PubMed
-
- Agid O, Siu CO, Potkin SG, et al. Meta-Regression Analysis of Placebo Response in Antipsychotic Trials, 1970–2010. Am J Psychiatry. 2013;170:1335–1344. - PubMed
-
- Mallinckrodt CH, Zhang L, Prucka WR, Millen BA. Signal Detection and Placebo Response in Schizophrenia: Parallels with Depression. Psychopharm Bull. 2010;43:53–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

