Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas
- PMID: 25322816
- PMCID: PMC4282692
- DOI: 10.1007/s00401-014-1352-5
Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas
Abstract
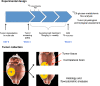
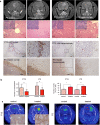
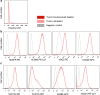
Anti-angiogenic therapy in glioblastoma (GBM) has unfortunately not led to the anticipated improvement in patient prognosis. We here describe how human GBM adapts to bevacizumab treatment at the metabolic level. By performing (13)C6-glucose metabolic flux analysis, we show for the first time that the tumors undergo metabolic re-programming toward anaerobic metabolism, thereby uncoupling glycolysis from oxidative phosphorylation. Following treatment, an increased influx of (13)C6-glucose was observed into the tumors, concomitant to increased lactate levels and a reduction of metabolites associated with the tricarboxylic acid cycle. This was confirmed by increased expression of glycolytic enzymes including pyruvate dehydrogenase kinase in the treated tumors. Interestingly, L-glutamine levels were also reduced. These results were further confirmed by the assessment of in vivo metabolic data obtained by magnetic resonance spectroscopy and positron emission tomography. Moreover, bevacizumab led to a depletion in glutathione levels indicating that the treatment caused oxidative stress in the tumors. Confirming the metabolic flux results, immunohistochemical analysis showed an up-regulation of lactate dehydrogenase in the bevacizumab-treated tumor core as well as in single tumor cells infiltrating the brain, which may explain the increased invasion observed after bevacizumab treatment. These observations were further validated in a panel of eight human GBM patients in which paired biopsy samples were obtained before and after bevacizumab treatment. Importantly, we show that the GBM adaptation to bevacizumab therapy is not mediated by clonal selection mechanisms, but represents an adaptive response to therapy.
Figures



References
-
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. - DOI - PMC - PubMed
-
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. - DOI - PMC - PubMed
-
- Baumgarten P, Brokinkel B, Zinke J, Zachskorn C, Ebel H, Albert FK, Stummer W, Plate KH, Harter PN, Hasselblatt M, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors VEGFR1 and VEGFR2 in primary and recurrent WHO grade III meningiomas. Histol Histopathol. 2013;28:1157–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

