The impact of anti-inflammatory cytokines on the pancreatic β-cell
- PMID: 25322830
- PMCID: PMC4292460
- DOI: 10.4161/19382014.2014.950547
The impact of anti-inflammatory cytokines on the pancreatic β-cell
Abstract
Considerable efforts have been invested to understand the mechanisms by which pro-inflammatory cytokines mediate the demise of β-cells in type 1 diabetes but much less attention has been paid to the role of anti-inflammatory cytokines as potential cytoprotective agents in these cells. Despite this, there is increasing evidence that anti-inflammatory molecules such as interleukin (IL)-4, IL-10 and IL-13 can exert a direct influence of β-cell function and viability and that the circulating levels of these cytokines may be reduced in type 1 diabetes. Thus, it seems possible that targeting of anti-inflammatory pathways might offer therapeutic potential in this disease. In the present review, we consider the evidence implicating IL-4, IL-10 and IL-13 as cytoprotective agents in the β-cell and discuss the receptor components and downstream signaling pathways that mediate these effects.
Keywords: GSIS, glucose-stimulated insulin secretion; IL, interleukin; Jak, janus kinase; NO, nitric oxide; PTP, protein tyrosine phosphatase; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; STAT3; STAT6; T1D, type 1 diabetes; Th, T-helper; interleukin-10; interleukin-13; interleukin-4; type 1 diabetes.
Figures
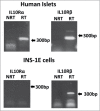

References
-
- Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009; 52:1143-51; PMID:19266182; http://dx.doi.org/10.1007/s00125-009-1276-0 - DOI - PubMed
-
- Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Del Prato S, et al. . Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA 2007; 104:5115-20; PMID:17360338; http://dx.doi.org/10.1073/pnas.0700442104 - DOI - PMC - PubMed
-
- Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H. Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol 2011; 33:45-55; PMID:20424841; http://dx.doi.org/10.1007/s00281-010-0207-y - DOI - PubMed
-
- Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med 2012; 2:a007682; PMID:22315719; http://dx.doi.org/10.1101/cshperspect.a007682 - DOI - PMC - PubMed
-
- Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol 2014; 25(1):80-92; PMID:24522639 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
