Cyanobacterial biofuels: new insights and strain design strategies revealed by computational modeling
- PMID: 25323065
- PMCID: PMC4180434
- DOI: 10.1186/s12934-014-0128-x
Cyanobacterial biofuels: new insights and strain design strategies revealed by computational modeling
Abstract
Background: Cyanobacteria are increasingly recognized as promising cell factories for the production of renewable biofuels and chemical feedstocks from sunlight, CO2, and water. However, most biotechnological applications of these organisms are still characterized by low yields. Increasing the production performance of cyanobacteria remains therefore a crucial step.
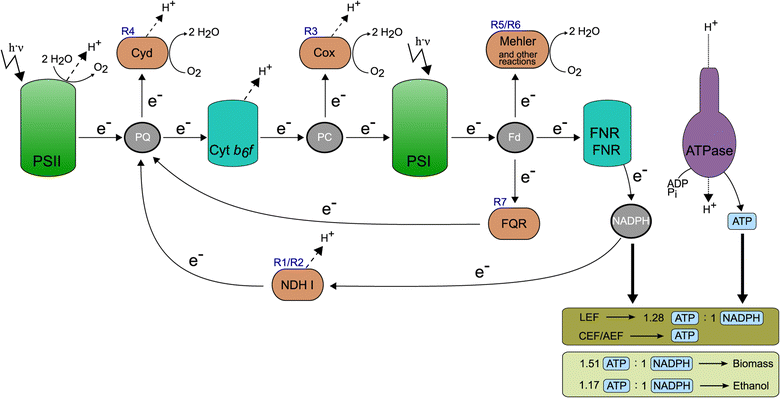
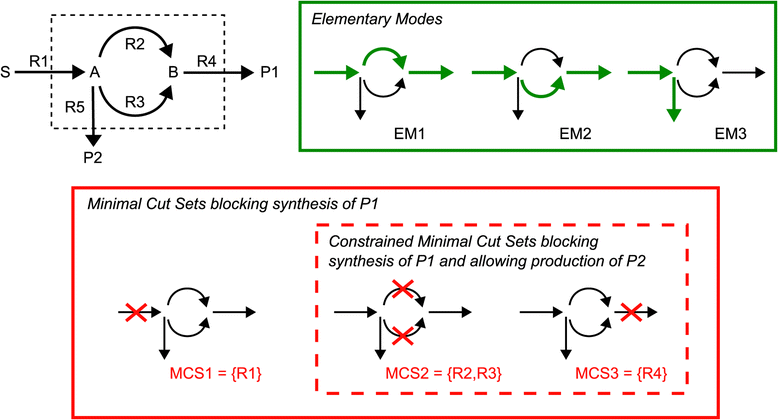
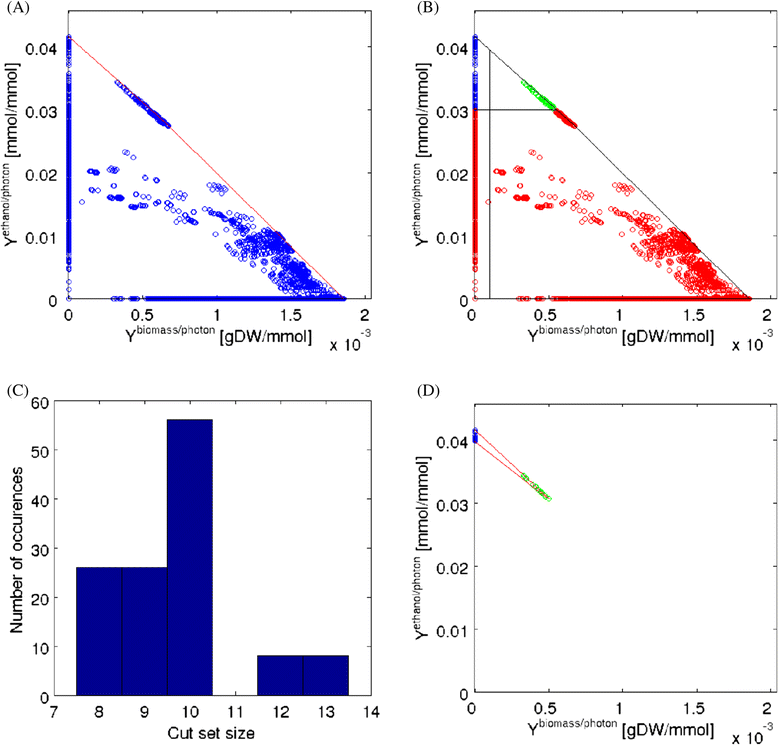
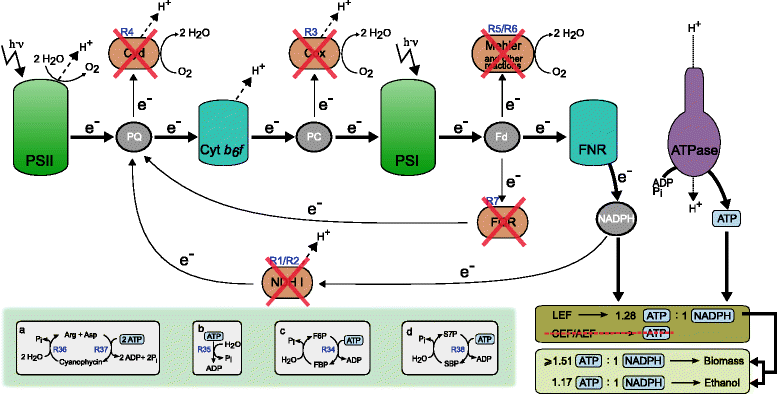
Results: In this work we use a stoichiometric network model of Synechocystis sp. PCC 6803 in combination with CASOP and minimal cut set analysis to systematically identify and characterize suitable strain design strategies for biofuel synthesis, specifically for ethanol and isobutanol. As a key result, improving upon other works, we demonstrate that higher-order knockout strategies exist in the model that lead to coupling of growth with high-yield biofuel synthesis under phototrophic conditions. Enumerating all potential knockout strategies (cut sets) reveals a unifying principle behind the identified strain designs, namely to reduce the ratio of ATP to NADPH produced by the photosynthetic electron transport chain. Accordingly, suitable knockout strategies seek to block cyclic and other alternate electron flows, such that ATP and NADPH are exclusively synthesized via the linear electron flow whose ATP/NADPH ratio is below that required for biomass synthesis. The products of interest are then utilized by the cell as sinks for reduction equivalents in excess. Importantly, the calculated intervention strategies do not rely on the assumption of optimal growth and they ensure that maintenance metabolism in the absence of light remains feasible. Our analyses furthermore suggest that a moderately increased ATP turnover, realized, for example, by ATP futile cycles or other ATP wasting mechanisms, represents a promising target to achieve increased biofuel yields.
Conclusion: Our study reveals key principles of rational metabolic engineering strategies in cyanobacteria towards biofuel production. The results clearly show that achieving obligatory coupling of growth and product synthesis in photosynthetic bacteria requires fundamentally different intervention strategies compared to heterotrophic organisms.
Figures

References
-
- Hess WR. Cyanobacterial genomics for ecology and biotechnology. Curr Opin Biotechnol. 2011;14(5):608–614. - PubMed
-
- Thajuddin N, Subramanian G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr Sci. 2005;89(1):47–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

