Neuropathology and genetics of cerebroretinal vasculopathies
- PMID: 25323666
- PMCID: PMC8029267
- DOI: 10.1111/bpa.12178
Neuropathology and genetics of cerebroretinal vasculopathies
Abstract
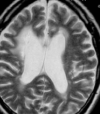
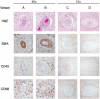
Cerebroretinal vasculopathy (CRV) and the related diseases hereditary endotheliopathy with retinopathy, neuropathy, and stroke (HERNS), hereditary vascular retinopathy (HVR) and hereditary systemic angiopathy (HSA) [subsequently combined as retinovasculopathy and cerebral leukodystrophy (RVCL)] are devastating autosomal-dominant disorders of early to middle-age onset presenting with a core constellation of neurologic and ophthalmologic findings. This family of diseases is linked by specific mutations targeting a core region of a gene. Frameshift mutations in the carboxyl-terminus of three prime exonuclease-1 (TREX1), the major mammalian 3' to 5' DNA exonuclease on chromosome 3p21.1-p21.3, result in a systemic vasculopathy that follows an approximately 5-year course leading to death secondary to progressive neurologic decline, with sometimes a more protracted course in HERNS. Neuropathological features include a fibrinoid vascular necrosis or thickened hyalinized vessels associated with white matter ischemia, necrosis and often striking dystrophic calcifications. Ultrastructural studies of the vessel walls often demonstrate unusual multilaminated basement membranes.
Keywords: CRV; HERNS; and stroke; cerebral microvascular disease; hereditary endotheliopathy with retinopathy; hereditary vascular retinopathy; nephropathy; ocular microvascular disease.
© 2014 International Society of Neuropathology.
Figures





References
-
- Chowdhury D, Beresford PJ, Zhu P, Zhang D, Sung J‐S, Demple B et al (2006) The exonuclease TREX1 is in the SET complex and acts in concert with NM23‐H1 to degrade DNA during granzyme A‐mediated cell death. Mol Cell 23:133–142. - PubMed
-
- Cohn AC, Kotschet K, Veitch A, Delatycki MB, McCombe MF (2005) Novel ophthalmological features in hereditary endotheliopathy with retinopathy, nephropathy and stroke syndrome. Clin Experiment Ophthalmol 33:181–183. - PubMed
-
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M et al (2006) Mutations in the gene encoding the 3“‐5” DNA exonuclease TREX1 cause Aicardi‐Goutières syndrome at the AGS1 locus. Nat Genet 38:917–920. - PubMed
-
- Grand MG, Kaine J, Fulling K, Atkinson J, Dowton SB, Farber M et al (1988) Cerebroretinal vasculopathy. A new hereditary syndrome. Ophthalmology 95:649–659. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

