Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity
- PMID: 25323693
- PMCID: PMC4245333
- DOI: 10.1038/cgt.2014.53
Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity
Abstract
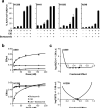
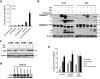
Delivery of suicide genes to solid tumors represents a promising tumor therapy strategy. However, slow or limited killing by suicide genes and ineffective targeting of the tumor has reduced effectiveness. We have adapted a suicide system based on an inducible caspase-9 (iC9) protein that is activated using a specific chemical inducer of dimerization (CID) for adenoviral-based delivery to lung tumors via mesenchymal stromal cells (MSCs). Four independent human non-small cell lung cancer (NSCLC) cell lines were transduced with adenovirus encoding iC9, and all underwent apoptosis when iC9 was activated by adding CID. However, there was a large variation in the percentage of cell killing induced by CID across the different lines. The least responsive cell lines were sensitized to apoptosis by combined inhibition of the proteasome using bortezomib. These results were extended to an in vivo model using human NSCLC xenografts. E1A-expressing MSCs replicated Ad.iC9 and delivered the virus to lung tumors in SCID mice. Treatment with CID resulted in some reduction of tumor growth, but addition of bortezomib led to greater reduction of tumor size. The enhanced apoptosis and anti-tumor effect of combining MSC-delivered Ad.iC9, CID and bortezomib appears to be due to increased stabilization of active caspase-3, as proteasomal inhibition increased the levels of cleaved caspase-9 and caspase-3. Knockdown of X-linked inhibitor of apoptosis protein (XIAP), a caspase inhibitor that targets active caspase-3 to the proteasome, also sensitized iC9-transduced cells to CID, suggesting that blocking the proteasome counteracts XIAP to permit apoptosis. Thus, MSC-based delivery of the iC9 suicide gene to human NSCLC effectively targets lung cancer cells for elimination. Combining this therapy with bortezomib, a drug that is otherwise inactive in this disease, further enhances the anti-tumor activity of this strategy.
Figures




References
-
- Duarte S, Carle G, Faneca H, de Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324(2):160–170. - PubMed
-
- Mohit E, Rafati S. Biological delivery approaches for gene therapy: Strategies to potentiate efficacy and enhance specificity. Mol Immunol. 2013;56(4):599–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

