The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble
- PMID: 25323859
- PMCID: PMC4282600
- DOI: 10.1161/CIRCRESAHA.116.303554
The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble
Abstract
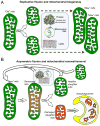
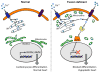
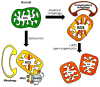
Mitochondrial research is experiencing a renaissance, in part, because of the recognition that these endosymbiotic descendants of primordial protobacteria seem to be pursuing their own biological agendas. Not only is mitochondrial metabolism required to produce most of the biochemical energy that supports their eukaryotic hosts (us) but mitochondria can actively (through apoptosis and programmed necrosis) or passively (through reactive oxygen species toxicity) drive cellular dysfunction or demise. The cellular mitochondrial collective autoregulates its population through biogenic renewal and mitophagic culling; mitochondrial fission and fusion, 2 components of mitochondrial dynamism, are increasingly recognized as playing central roles as orchestrators of these processes. Mitochondrial dynamism is rare in striated muscle cells, so cardiac-specific genetic manipulation of mitochondrial fission and fusion factors has proven useful for revealing noncanonical functions of mitochondrial dynamics proteins. Here, we review newly described functions of mitochondrial fusion/fission proteins in cardiac mitochondrial quality control, cell death, calcium signaling, and cardiac development. A mechanistic conceptual paradigm is proposed in which cell death and selective organelle culling are not distinct processes, but are components of a unified and integrated quality control mechanism that exerts different effects when invoked to different degrees, depending on pathophysiological context. This offers a plausible explanation for seemingly paradoxical expression of mitochondrial dynamics and death factors in cardiomyocytes wherein mitochondrial morphometric remodeling does not normally occur and the ability to recover from cell suicide is severely limited.
Keywords: apoptosis; autophagy; mitochondria; mitochondrial dynamics.
© 2014 American Heart Association, Inc.
Figures




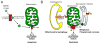
References
-
- Lewin R. The unmasking of mitochondrial Eve. Science. 1987;238:24–26. - PubMed
-
- Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. - PubMed
-
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

