Bovine posterior limbus: an evaluation of an alternative source for corneal endothelial and trabecular meshwork stem/progenitor cells
- PMID: 25323922
- PMCID: PMC4334470
- DOI: 10.1089/scd.2014.0257
Bovine posterior limbus: an evaluation of an alternative source for corneal endothelial and trabecular meshwork stem/progenitor cells
Abstract
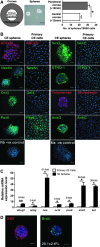
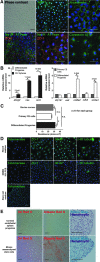
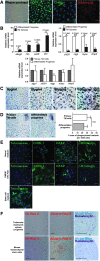
A growing body of evidence has revealed that stem-like cells in the posterior limbus of the eye between the corneal endothelium (CE) and trabecular meshwork (TM) may be able to rejuvenate these tissues in disease. However, these cells have not been clearly defined and we have named them PET cells (progenitor cells of the endothelium and trabeculum). A good and inexpensive animal model for PET cells is lacking, so we investigated bovine eyes as an effective large tissue source. We showed the presence of stem/progenitor cells in the bovine CE, transition zone, and TM in situ. Floating spheres cultured from the CE and TM showed similar stem cell marker expression patterns. Both the CE and TM spheres were bipotent and highly proliferative, but with limited secondary sphere-forming capability. They were highly prone to differentiate back into the cell type of their tissue of origin. It is speculated that the PET cells become more tissue-specific as they migrate away from their niche. Here, we showed that PET cells are present in the posterior limbus of bovine eyes and that they can be successfully cultured and expanded. PET cells represent an attractive target for developing new treatments to regenerate both the CE and TM, thereby reducing the requirement for donor tissue for corneal transplant and invasive treatments for glaucomatous patients.
Figures




References
-
- Joyce NC. (2003). Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 22:359–389 - PubMed
-
- Grierson I. and Hogg P. (1995). The proliferative and migratory activities of trabecular meshwork cells. Prog Retin Eye Res 15:33–67
-
- Alvarado J, Murphy C, Polansky J. and Juster R. (1981). Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci 21:714–727 - PubMed
-
- Alvarado J, Murphy C. and Juster R. (1984). Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology 91:564–579 - PubMed
-
- Ehlers JP, Shah CP, Fenton GL, Hoskins EN, Shelsta HN, Friedberg MA. and Rapuano CJ, eds. (2008). The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

