The cellular origins of the outer retinal bands in optical coherence tomography images
- PMID: 25324288
- PMCID: PMC4261632
- DOI: 10.1167/iovs.14-14907
The cellular origins of the outer retinal bands in optical coherence tomography images
Abstract
Purpose: To test the recently proposed hypothesis that the second outer retinal band, observed in clinical OCT images, originates from the inner segment ellipsoid, by measuring: (1) the thickness of this band within single cone photoreceptors, and (2) its respective distance from the putative external limiting membrane (band 1) and cone outer segment tips (band 3).
Methods: Adaptive optics-optical coherence tomography images were acquired from four subjects without known retinal disease. Images were obtained at foveal (2°) and perifoveal (5°) locations. Cone photoreceptors (n = 9593) were identified and segmented in three dimensions using custom software. Features corresponding to bands 1, 2, and 3 were automatically identified. The thickness of band 2 was assessed in each cell by fitting the longitudinal reflectance profile of the band with a Gaussian function. Distances between bands 1 and 2, and between 2 and 3, respectively, were also measured in each cell. Two independent calibration techniques were employed to determine the depth scale (physical length per pixel) of the imaging system.
Results: When resolved within single cells, the thickness of band 2 is a factor of three to four times narrower than in corresponding clinical OCT images. The distribution of band 2 thickness across subjects and eccentricities had a modal value of 4.7 μm, with 48% of the cones falling between 4.1 and 5.2 μm. No significant differences were found between cells in the fovea and perifovea. The distance separating bands 1 and 2 was found to be larger than the distance between bands 2 and 3, across subjects and eccentricities, with a significantly larger difference at 5° than 2°.
Conclusions: On the basis of these findings, we suggest that ascription of the outer retinal band 2 to the inner segment ellipsoid is unjustified, because the ellipsoid is both too thick and proximally located to produce the band.
Keywords: adaptive optics; morphometry; nomenclature; optical coherence tomography; photoreceptor morphology.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures
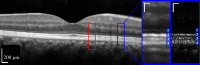





 , defined by the distance between peak of the band 1 (ELM) reflection and the peak of the band 2 reflection. Because no subpixel fitting of peaks was employed, precision was limited by the 0.74 μm (RMS) axial quantization error.
, defined by the distance between peak of the band 1 (ELM) reflection and the peak of the band 2 reflection. Because no subpixel fitting of peaks was employed, precision was limited by the 0.74 μm (RMS) axial quantization error.
 , defined by the distance between peak of the band 2 reflection and the peak of the band 3 reflection. Because no subpixel fitting of peaks was employed, precision was limited by the 0.74 μm (RMS) axial quantization error.
, defined by the distance between peak of the band 2 reflection and the peak of the band 3 reflection. Because no subpixel fitting of peaks was employed, precision was limited by the 0.74 μm (RMS) axial quantization error.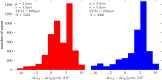
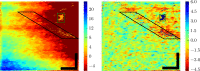
 at 2.0° (left) and 5.0° (right), for all four subjects. At both eccentricities
at 2.0° (left) and 5.0° (right), for all four subjects. At both eccentricities
 for most cones, with average differentials of + 2.5 and + 3.3 μm at 2.0° and 5.0°, respectively. Thus, band 2 lies approximately between band 1 (ELM) and band 3, but slightly closer to the latter.
for most cones, with average differentials of + 2.5 and + 3.3 μm at 2.0° and 5.0°, respectively. Thus, band 2 lies approximately between band 1 (ELM) and band 3, but slightly closer to the latter.
Comment in
-
Outer Retinal Bands.Invest Ophthalmol Vis Sci. 2015 Apr;56(4):2505-6. doi: 10.1167/iovs.15-16456. Invest Ophthalmol Vis Sci. 2015. PMID: 26066596 No abstract available.
-
Author Response: Outer Retinal Bands.Invest Ophthalmol Vis Sci. 2015 Apr;56(4):2507-10. doi: 10.1167/iovs.15-16756. Invest Ophthalmol Vis Sci. 2015. PMID: 26066597 Free PMC article. No abstract available.
References
-
- Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995; 113: 586–596. - PubMed
-
- Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000; 107: 2278–2282. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

