Cardiac troponin I Pro82Ser variant induces diastolic dysfunction, blunts β-adrenergic response, and impairs myofilament cooperativity
- PMID: 25324519
- PMCID: PMC4297775
- DOI: 10.1152/japplphysiol.00463.2014
Cardiac troponin I Pro82Ser variant induces diastolic dysfunction, blunts β-adrenergic response, and impairs myofilament cooperativity
Abstract
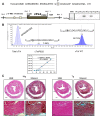
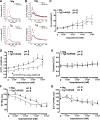
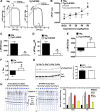
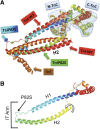
Troponin I (TnI) variant Pro82Ser (cTnIP82S) was initially considered a disease-causing mutation; however, later studies suggested the contrary. We tested the hypothesis of whether a causal link exists between cTnIP82S and cardiac structural and functional remodeling, such as during aging or chronic pressure overload. A cardiac-specific transgenic (Tg) mouse model of cTnIP82S was created to test this hypothesis. During aging, Tg cTnIP82S displayed diastolic dysfunction, characterized by longer isovolumetric relaxation time, and impaired ejection and relaxation time. In young, Tg mice in vivo pressure-volume loops and intact trabecular preparations revealed normal cardiac contractility at baseline. However, upon β-adrenergic stimulation, a blunted contractile reserve and no hastening in left ventricle relaxation were evident in vivo, whereas, in isolated muscles, Ca(2+) transient amplitude isoproterenol dose-response was blunted. In addition, when exposed to chronic pressure overload, Tg mice show exacerbated hypertrophy and decreased contractility compared with age-matched non-Tg littermates. At the molecular level, this mutation significantly impairs myofilament cooperative activation. Importantly, this occurs in the absence of alterations in TnI or myosin-binding protein C phosphorylation. The cTnIP82S variant occurs near a region of interactions with troponin T; therefore, structural changes in this region could explain its meaningful effects on myofilament cooperativity. Our data indicate that cTnIP82S mutation modifies age-dependent diastolic dysfunction and impairs overall contractility after β-adrenergic stimulation or chronic pressure overload. Thus cTnIP82S variant should be regarded as a disease-modifying factor for dysfunction and adverse remodeling with aging and chronic pressure overload.
Keywords: cardiac troponin I mutation; diastolic dysfunction; hypertrophy; transgenic mouse.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Biesiadecki BJ, Schneider KL, Yu ZB, Chong SM, Jin JP. An R111C polymorphism in wild turkey cardiac troponin I accompanying the dilated cardiomyopathy-related abnormal splicing variant of cardiac troponin T with potentially compensatory effects. J Biol Chem 279: 13825–13832, 2004. - PubMed
-
- Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol 292: H318–H325, 2007. - PubMed
-
- Blanchard E, Seidman C, Seidman JG, LeWinter M, Maughan D. Altered crossbridge kinetics in the αMHC(403/+) mouse model of familial hypertrophic cardiomyopathy. Circ Res 84: 475–483, 1999. - PubMed
-
- Brown AT, Frazier A, Dennison CR, Hill MN, Post WS, Robinson JC, Murphy AM. A Non-synonymous variant of the cardiac troponin I gene is associated with enhanced cardiac hypertrophy response to hypertension in young black men (Abstract). In: AHA Scientific Basis of Heart Failure in Children. Dallas, TX: American Heart Association, 2008.
-
- Cardim N, Perrot A, Ferreira T, Pereira A, Osterziel KJ, Reis RP, Correia JF. Usefulness of Doppler myocardial imaging for identification of mutation carriers of familial hypertrophic cardiomyopathy. Am J Cardiol 90: 128–132, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

