A nontranscriptional role for Oct4 in the regulation of mitotic entry
- PMID: 25324523
- PMCID: PMC4226071
- DOI: 10.1073/pnas.1417518111
A nontranscriptional role for Oct4 in the regulation of mitotic entry
Abstract
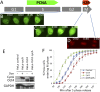
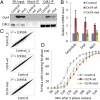
Rapid progression through the cell cycle and a very short G1 phase are defining characteristics of embryonic stem cells. This distinct cell cycle is driven by a positive feedback loop involving Rb inactivation and reduced oscillations of cyclins and cyclin-dependent kinase (Cdk) activity. In this setting, we inquired how ES cells avoid the potentially deleterious consequences of premature mitotic entry. We found that the pluripotency transcription factor Oct4 (octamer-binding transcription factor 4) plays an unappreciated role in the ES cell cycle by forming a complex with cyclin-Cdk1 and inhibiting Cdk1 activation. Ectopic expression of Oct4 or a mutant lacking transcriptional activity recapitulated delayed mitotic entry in HeLa cells. Reduction of Oct4 levels in ES cells accelerated G2 progression, which led to increased chromosomal missegregation and apoptosis. Our data demonstrate an unexpected nontranscriptional function of Oct4 in the regulation of mitotic entry.
Keywords: CDC25; Cdk1; Oct4; mitotic entry; pluripotent stem cells.
Conflict of interest statement
Conflict of interest statement: G.Q.D. is a member of the scientific advisory boards of and holds stock in or receives consulting fees from the following companies: Johnson & Johnson, Verastem, Epizyme, iPierian, Solasia KK, and MPM Capital, LLP.
Figures





References
-
- Becker KA, et al. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209(3):883–893. - PubMed
-
- Savatier P, Huang S, Szekely L, Wiman KG, Samarut J. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene. 1994;9(3):809–818. - PubMed
-
- Stead E, et al. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21(54):8320–8333. - PubMed
-
- Dulić V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257(5078):1958–1961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM026875/GM/NIGMS NIH HHS/United States
- R01 GM026875/GM/NIGMS NIH HHS/United States
- R24DK092760/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P50 NS040828/NS/NINDS NIH HHS/United States
- NIH-P30-HD18655/HD/NICHD NIH HHS/United States
- U01 HL100001/HL/NHLBI NIH HHS/United States
- R24 DK092760/DK/NIDDK NIH HHS/United States
- RC4GM096319/GM/NIGMS NIH HHS/United States
- R01 GM039023/GM/NIGMS NIH HHS/United States
- K99 HD061981/HD/NICHD NIH HHS/United States
- K99HD061981/HD/NICHD NIH HHS/United States
- NIH-P50-NS40828/NS/NINDS NIH HHS/United States
- UO1-HL100001/HL/NHLBI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01GM039023/GM/NIGMS NIH HHS/United States
- T32HL007623/HL/NHLBI NIH HHS/United States
- RC4 GM096319/GM/NIGMS NIH HHS/United States
- T32 HL007623/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

