Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes
- PMID: 25324711
- PMCID: PMC4199458
- DOI: 10.1186/1617-9625-12-17
Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes
Abstract
Background: Electronic nicotine delivery systems (ENDS) present an emerging issue for tobacco control and data on product use behaviors are limited.
Methods: Participants (N = 38 enrolled; N = 16 compliant) completed three lab visits over 5 days and were asked to abstain from regular cigarettes for 72 hours in favor of ENDS (Smoke 51 TRIO - 3 piece, First Generation with 11 mg/ml filters). Lab visits included measurement of exhaled carbon monoxide (CO) and salivary cotinine concentration, questionnaire measures of regular cigarette craving after the 72 hour abstinence, and subjective product effects. Participants used a topography device to record puff volume, duration, flow rate, and inter-puff interval.
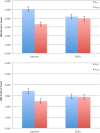
Results: Analyses revealed significant differences across products in puff count, average volume, total volume and inter-puff interval, with ENDS broadly showing a more intensive smoking pattern. Cigarette craving scores dropped significantly after smoking regular cigarettes, but not ENDS (p = .001), and subjective measures showed ENDS rated less favorably. CO boost, after ENDS use, decreased significantly (p < .001), and saliva cotinine significantly dropped between visits 1 and 3 (p < 0.001) after ENDS use relative to after cigarette smoking. For compliant and non-compliant participants, there was an average 82.0% [V1 - 16.1 cpd; V3 - 2.9 cpd] and average 73.9% [V1 - 20.3 cpd; V3 - 5.3 cpd] reduction in regular cigarette use per day during the ENDS trial period, respectively.
Conclusions: The ENDS were smoked more intensively than own brand cigarettes, but delivered significantly less nicotineand were less satisfying. These findings have implications for the viability of certain ENDS as alternatives to cigarettes.
Keywords: Behavior; Smoking; Subjective effects.
Figures
Similar articles
-
Changes in puffing topography and subjective effects over a 2-week period in e-cigarette naïve smokers: Effects of device type and nicotine concentrations.Addict Behav. 2021 Jul;118:106909. doi: 10.1016/j.addbeh.2021.106909. Epub 2021 Mar 11. Addict Behav. 2021. PMID: 33756301 Clinical Trial.
-
Associated Changes in E-cigarette Puff Duration and Cigarettes Smoked per Day.Nicotine Tob Res. 2021 Mar 19;23(4):760-764. doi: 10.1093/ntr/ntaa211. Nicotine Tob Res. 2021. PMID: 33049064 Free PMC article.
-
Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes.Addict Behav. 2015 Sep;48:1-4. doi: 10.1016/j.addbeh.2015.04.003. Epub 2015 Apr 16. Addict Behav. 2015. PMID: 25930009 Free PMC article.
-
Actual Use Behavior Assessment of a Novel Puff Recording Electronic Nicotine Delivery System: Observation Study.JMIR Form Res. 2023 Feb 8;7:e43175. doi: 10.2196/43175. JMIR Form Res. 2023. PMID: 36592426 Free PMC article.
-
Smoking behaviour and compensation: a review of the literature.Psychopharmacology (Berl). 1999 Jul;145(1):1-20. doi: 10.1007/s002130051027. Psychopharmacology (Berl). 1999. PMID: 10445368 Review.
Cited by
-
Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked.Int J Environ Res Public Health. 2020 May 6;17(9):3225. doi: 10.3390/ijerph17093225. Int J Environ Res Public Health. 2020. PMID: 32384697 Free PMC article.
-
Impact of e-liquid flavors on e-cigarette vaping behavior.Drug Alcohol Depend. 2018 Aug 1;189:42-48. doi: 10.1016/j.drugalcdep.2018.04.032. Epub 2018 May 31. Drug Alcohol Depend. 2018. PMID: 29879680 Free PMC article.
-
The Influence of a Mouthpiece-Based Topography Measurement Device on Electronic Cigarette User's Plasma Nicotine Concentration, Heart Rate, and Subjective Effects Under Directed and Ad Libitum Use Conditions.Nicotine Tob Res. 2017 Apr 1;19(4):469-476. doi: 10.1093/ntr/ntw174. Nicotine Tob Res. 2017. PMID: 27613914 Free PMC article.
-
Qualitative analysis of young adult ENDS users' expectations and experiences.BMJ Open. 2017 Mar 7;7(3):e014990. doi: 10.1136/bmjopen-2016-014990. BMJ Open. 2017. PMID: 28270392 Free PMC article.
-
Examining Daily Electronic Cigarette Puff Topography Among Established and Nonestablished Cigarette Smokers in their Natural Environment.Nicotine Tob Res. 2018 Sep 4;20(10):1283-1288. doi: 10.1093/ntr/ntx222. Nicotine Tob Res. 2018. PMID: 29059416 Free PMC article.
References
-
- Westenberger BJ. Evaluation of e-cigarettes. St. Louis: US Food and Drug Administration, Center for Drug Evaluation and Research. 2009. http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous