Chemokine receptors as important regulators of pathogenesis during arboviral encephalitis
- PMID: 25324719
- PMCID: PMC4179766
- DOI: 10.3389/fncel.2014.00264
Chemokine receptors as important regulators of pathogenesis during arboviral encephalitis
Abstract
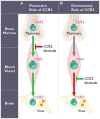
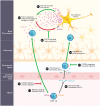
The central nervous system (CNS) is a highly complex network comprising long-lived neurons and glial cells. Accordingly, numerous mechanisms have evolved to tightly regulate the initiation of inflammatory responses within the brain. Under neuroinflammatory conditions, as in the case of viral encephalitides, the infiltration of leukocytes is often required for efficient viral clearance and recovery. The orchestration of leukocyte migration into the inflamed CNS is largely coordinated by a large family of chemotactic cytokines and their receptors. In this review, we will summarize our current understanding of how chemokines promote protection or pathogenesis during arbovirus induced encephalitis, focusing on neurotropic flaviviruses and alphaviruses. Furthermore, we will highlight the latest developments in chemokine and chemokine receptor based drugs that could have potential as therapeutics and have been shown to play a pivotal role in shaping the outcome of disease.
Keywords: alphaviruses; antagonists; chemokine receptors; flaviviruses; leukocyte infiltration.
Figures

References
-
- Bachelerie F., Ben-Baruch A., Burkhardt A. M., Combadiere C., Farber J. M., Graham G. J., et al. (2013). International Union of Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 66 1–79 10.1124/pr.113.007724 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

