The natural history of sound localization in mammals--a story of neuronal inhibition
- PMID: 25324726
- PMCID: PMC4181121
- DOI: 10.3389/fncir.2014.00116
The natural history of sound localization in mammals--a story of neuronal inhibition
Abstract
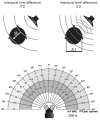
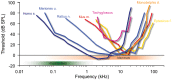
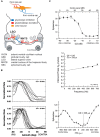
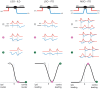
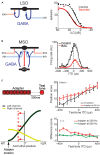
Our concepts of sound localization in the vertebrate brain are widely based on the general assumption that both the ability to detect air-borne sounds and the neuronal processing are homologous in archosaurs (present day crocodiles and birds) and mammals. Yet studies repeatedly report conflicting results on the neuronal circuits and mechanisms, in particular the role of inhibition, as well as the coding strategies between avian and mammalian model systems. Here we argue that mammalian and avian phylogeny of spatial hearing is characterized by a convergent evolution of hearing air-borne sounds rather than by homology. In particular, the different evolutionary origins of tympanic ears and the different availability of binaural cues in early mammals and archosaurs imposed distinct constraints on the respective binaural processing mechanisms. The role of synaptic inhibition in generating binaural spatial sensitivity in mammals is highlighted, as it reveals a unifying principle of mammalian circuit design for encoding sound position. Together, we combine evolutionary, anatomical and physiological arguments for making a clear distinction between mammalian processing mechanisms and coding strategies and those of archosaurs. We emphasize that a consideration of the convergent nature of neuronal mechanisms will significantly increase the explanatory power of studies of spatial processing in both mammals and birds.
Keywords: GABA; LSO; MSO; archosaurs; binaural hearing; birds; evolution; glycine.
Figures



References
-
- Blauert J. (1997). Spatial Hearing: The Psychophysics of Human Sound Localization. Cambridge: MIT Press
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

