Alterations in thin filament length during postnatal skeletal muscle development and aging in mice
- PMID: 25324783
- PMCID: PMC4178374
- DOI: 10.3389/fphys.2014.00375
Alterations in thin filament length during postnatal skeletal muscle development and aging in mice
Abstract
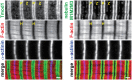
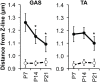
The lengths of the sarcomeric thin filaments vary in a skeletal muscle-specific manner and help specify the physiological properties of skeletal muscle. Since the extent of overlap between the thin and thick filaments determines the amount of contractile force that a sarcomere can actively produce, thin filament lengths are accurate predictors of muscle-specific sarcomere length-tension relationships and sarcomere operating length ranges. However, the striking uniformity of thin filament lengths within sarcomeres, specified during myofibril assembly, has led to the widely held assumption that thin filament lengths remain constant throughout an organism's lifespan. Here, we rigorously tested this assumption by using computational super-resolution image analysis of confocal fluorescence images to explore the effects of postnatal development and aging on thin filament length in mice. We found that thin filaments shorten in postnatal tibialis anterior (TA) and gastrocnemius muscles between postnatal days 7 and 21, consistent with the developmental program of myosin heavy chain (MHC) gene expression in this interval. By contrast, thin filament lengths in TA and extensor digitorum longus (EDL) muscles remained constant between 2 mo and 2 yr of age, while thin filament lengths in soleus muscle became shorter, suggestive of a slow-muscle-specific mechanism of thin filament destabilization associated with aging. Collectively, these data are the first to show that thin filament lengths change as part of normal skeletal muscle development and aging, motivating future investigations into the cellular and molecular mechanisms underlying thin filament adaptation across the lifespan.
Keywords: actin; mouse; myofibril; myofilament; sarcomere; tropomodulin.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

