Immunity to RSV in Early-Life
- PMID: 25324843
- PMCID: PMC4179512
- DOI: 10.3389/fimmu.2014.00466
Immunity to RSV in Early-Life
Abstract
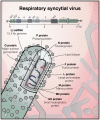
Respiratory Syncytial Virus (RSV) is the commonest cause of severe respiratory infection in infants, leading to over 3 million hospitalizations and around 66,000 deaths worldwide each year. RSV bronchiolitis predominantly strikes apparently healthy infants, with age as the principal risk factor for severe disease. The differences in the immune response to RSV in the very young are likely to be key to determining the clinical outcome of this common infection. Remarkable age-related differences in innate cytokine responses follow recognition of RSV by numerous pattern recognition receptors, and the importance of this early response is supported by polymorphisms in many early innate genes, which associate with bronchiolitis. In the absence of strong, Th1 polarizing signals, infants develop T cell responses that can be biased away from protective Th1 and cytotoxic T cell immunity toward dysregulated, Th2 and Th17 polarization. This may contribute not only to the initial inflammation in bronchiolitis, but also to the long-term increased risk of developing wheeze and asthma later in life. An early-life vaccine for RSV will need to overcome the difficulties of generating a protective response in infants, and the proven risks associated with generating an inappropriate response. Infantile T follicular helper and B cell responses are immature, but maternal antibodies can afford some protection. Thus, maternal vaccination is a promising alternative approach. However, even in adults adaptive immunity following natural infection is poorly protective, allowing re-infection even with the same strain of RSV. This gives us few clues as to how effective vaccination could be achieved. Challenges remain in understanding how respiratory immunity matures with age, and the external factors influencing its development. Determining why some infants develop bronchiolitis should lead to new therapies to lessen the clinical impact of RSV and aid the rational design of protective vaccines.
Keywords: RSV; bronchiolitis; mucosal immunology; neonatal; respiratory; viral.
Figures

References
-
- Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent. I. Isolation, properties and characterization. Am J Hyg (1957) 66:281–90 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

