EPO Mediates Neurotrophic, Neuroprotective, Anti-Oxidant, and Anti-Apoptotic Effects via Downregulation of miR-451 and miR-885-5p in SH-SY5Y Neuron-Like Cells
- PMID: 25324845
- PMCID: PMC4179732
- DOI: 10.3389/fimmu.2014.00475
EPO Mediates Neurotrophic, Neuroprotective, Anti-Oxidant, and Anti-Apoptotic Effects via Downregulation of miR-451 and miR-885-5p in SH-SY5Y Neuron-Like Cells
Abstract
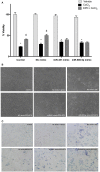
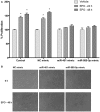
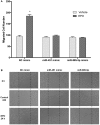
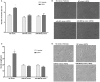
Erythropoietin (EPO) is a neuroprotective cytokine, which has been applied in several animal models presenting neurological disorders. One of the proposed modes of action resulting in neuroprotection is post-transcriptional gene expression regulation. This directly brings to mind microRNAs (miRNAs), which are small non-coding RNAs that regulate gene expression at the post-transcriptional level. It has not yet been evaluated whether miRNAs participate in the biological effects of EPO or whether it, inversely, modulates specific miRNAs in neuronal cells. In this study, we employed miRNA and mRNA arrays to identify how EPO exerts its biological function. Notably, miR-451 and miR-885-5p are downregulated in EPO-treated SH-SY5Y neuronal-like cells. Accordingly, target prediction and transcriptome analysis of cells treated with EPO revealed an alteration of the expression of genes involved in apoptosis, cell survival, proliferation, and migration. Low expression of miRNAs in SH-SY5Y was correlated with high expression of their target genes, vascular endothelial growth factor A, matrix metallo peptidase 9 (MMP9), cyclin-dependent kinase 2 (CDK2), erythropoietin receptor, Mini chromosome maintenance complex 5 (MCM5), B-cell lymphoma 2 (BCL2), and Galanin (GAL). Cell viability, apoptosis, proliferation, and migration assays were carried out for functional analysis after transfection with miRNA mimics, which inhibited some biological actions of EPO such as neuroprotection, anti-oxidation, anti-apoptosis, and migratory effects. In this study, we report for the first time that EPO downregulates the expression of miRNAs (miR-451 and miR-885-5p) in SH-SY5Y neuronal-like cells. The correlation between the over-expression of miRNAs and the decrease in EPO-mediated biological effects suggests that miR-451 and miR-885-5p may play a key role in the mediation of biological function.
Keywords: apoptosis; cell death; erythropoietin; miRNAs; migration; neurite outgrowth; oxidative stress; proliferation.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

