Genetic architecture of sex determination in fish: applications to sex ratio control in aquaculture
- PMID: 25324858
- PMCID: PMC4179683
- DOI: 10.3389/fgene.2014.00340
Genetic architecture of sex determination in fish: applications to sex ratio control in aquaculture
Abstract
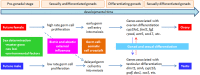
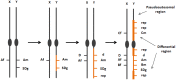
Controlling the sex ratio is essential in finfish farming. A balanced sex ratio is usually good for broodstock management, since it enables to develop appropriate breeding schemes. However, in some species the production of monosex populations is desirable because the existence of sexual dimorphism, primarily in growth or first time of sexual maturation, but also in color or shape, can render one sex more valuable. The knowledge of the genetic architecture of sex determination (SD) is convenient for controlling sex ratio and for the implementation of breeding programs. Unlike mammals and birds, which show highly conserved master genes that control a conserved genetic network responsible for gonad differentiation (GD), a huge diversity of SD mechanisms has been reported in fish. Despite theory predictions, more than one gene is in many cases involved in fish SD and genetic differences have been observed in the GD network. Environmental factors also play a relevant role and epigenetic mechanisms are becoming increasingly recognized for the establishment and maintenance of the GD pathways. Although major genetic factors are frequently involved in fish SD, these observations strongly suggest that SD in this group resembles a complex trait. Accordingly, the application of quantitative genetics combined with genomic tools is desirable to address its study and in fact, when applied, it has frequently demonstrated a multigene trait interacting with environmental factors in model and cultured fish species. This scenario has notable implications for aquaculture and, depending upon the species, from chromosome manipulation or environmental control techniques up to classical selection or marker assisted selection programs, are being applied. In this review, we selected four relevant species or fish groups to illustrate this diversity and hence the technologies that can be used by the industry for the control of sex ratio: turbot and European sea bass, two reference species of the European aquaculture, and salmonids and tilapia, representing the fish for which there are well established breeding programs.
Keywords: aquaculture; fish; genetic architecture; sex determination; sex ratio.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

