Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: a phase II study by the Japan Adult Leukemia Study Group
- PMID: 25325302
- PMCID: PMC4220650
- DOI: 10.1038/bcj.2014.72
Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: a phase II study by the Japan Adult Leukemia Study Group
Abstract
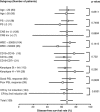
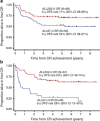
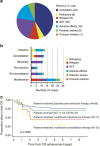
The superiority of the pediatric protocol for adolescents with acute lymphoblastic leukemia (ALL) has already been demonstrated, however, its efficacy in young adults remains unclear. The ALL202-U protocol was conducted to examine the efficacy and feasibility of a pediatric protocol in adolescents and young adults (AYAs) with BCR-ABL-negative ALL. Patients aged 15-24 years (n=139) were treated with the same protocol used for pediatric B-ALL. The primary objective of this study was to assess the disease-free survival (DFS) rate and its secondary aims were to assess toxicity, the complete remission (CR) rate and the overall survival (OS) rate. The CR rate was 94%. The 5-year DFS and OS rates were 67% (95% confidence interval (CI) 58-75%) and 73% (95% CI 64-80%), respectively. Severe adverse events were observed at a frequency that was similar to or lower than that in children treated with the same protocol. Only insufficient maintenance therapy significantly worsened the DFS (hazard ratio 5.60, P<0.001). These results indicate that this protocol may be a feasible and highly effective treatment for AYA with BCR-ABL-negative ALL.
Figures

References
-
- Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–1309. - PubMed
-
- Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980 s to the early 21st century. Blood. 2009;113:1408–1411. - PubMed
-
- Boissel N, Auclerc MF, Lheritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. - PubMed
-
- Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48:254–261. - PubMed
-
- Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

