Proteomic and biochemical analyses show a functional network of proteins involved in antioxidant defense of the Arabidopsis anp2anp3 double mutant
- PMID: 25325904
- PMCID: PMC4423761
- DOI: 10.1021/pr500588c
Proteomic and biochemical analyses show a functional network of proteins involved in antioxidant defense of the Arabidopsis anp2anp3 double mutant
Abstract
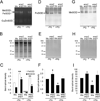
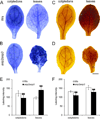
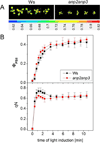
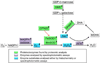
Disentanglement of functional complexity associated with plant mitogen-activated protein kinase (MAPK) signaling has benefited from transcriptomic, proteomic, phosphoproteomic, and genetic studies. Published transcriptomic analysis of a double homozygous recessive anp2anp3 mutant of two MAPK kinase kinase (MAPKKK) genes called Arabidopsis thaliana Homologues of Nucleus- and Phragmoplast-localized Kinase 2 (ANP2) and 3 (ANP3) showed the upregulation of stress-related genes. In this study, a comparative proteomic analysis of anp2anp3 mutant against its respective Wassilevskaja ecotype (Ws) wild type background is provided. Such differential proteomic analysis revealed overabundance of core enzymes such as FeSOD1, MnSOD, DHAR1, and FeSOD1-associated regulatory protein CPN20, which are involved in the detoxification of reactive oxygen species in the anp2anp3 mutant. The proteomic results were validated at the level of single protein abundance by Western blot analyses and by quantitative biochemical determination of antioxidant enzymatic activities. Finally, the functional network of proteins involved in antioxidant defense in the anp2anp3 mutant was physiologically linked with the increased resistance of mutant seedlings against paraquat treatment.
Keywords: ANP2; ANP3; Arabidopsis; antioxidant defense; mitogen-activated protein kinase kinase kinase; oxidative stress; proteomics; signaling.
Figures
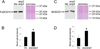
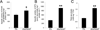

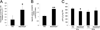

Similar articles
-
Stress signaling in response to polycyclic aromatic hydrocarbon exposure in Arabidopsis thaliana involves a nucleoside diphosphate kinase, NDPK-3.Planta. 2015 Jan;241(1):95-107. doi: 10.1007/s00425-014-2161-8. Epub 2014 Sep 16. Planta. 2015. PMID: 25224398
-
Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization.Plant Cell. 2010 Mar;22(3):755-71. doi: 10.1105/tpc.109.071746. Epub 2010 Mar 9. Plant Cell. 2010. PMID: 20215588 Free PMC article.
-
MKK6 Functions in Two Parallel MAP Kinase Cascades in Immune Signaling.Plant Physiol. 2018 Nov;178(3):1284-1295. doi: 10.1104/pp.18.00592. Epub 2018 Sep 5. Plant Physiol. 2018. PMID: 30185442 Free PMC article.
-
Quantitative Proteomic Analysis of ER Stress Response Reveals both Common and Specific Features in Two Contrasting Ecotypes of Arabidopsis thaliana.Int J Mol Sci. 2020 Dec 21;21(24):9741. doi: 10.3390/ijms21249741. Int J Mol Sci. 2020. PMID: 33371194 Free PMC article.
-
The Arabidopsis cytosolic proteome: the metabolic heart of the cell.Front Plant Sci. 2014 Feb 5;5:21. doi: 10.3389/fpls.2014.00021. eCollection 2014. Front Plant Sci. 2014. PMID: 24550929 Free PMC article. Review.
Cited by
-
ACT2.6: Global Gene Coexpression Network in Arabidopsis thaliana Using WGCNA.Genes (Basel). 2025 Feb 23;16(3):258. doi: 10.3390/genes16030258. Genes (Basel). 2025. PMID: 40149410 Free PMC article.
-
Cellular reprogramming through mitogen-activated protein kinases.Front Plant Sci. 2015 Oct 29;6:940. doi: 10.3389/fpls.2015.00940. eCollection 2015. Front Plant Sci. 2015. PMID: 26579181 Free PMC article. Review.
-
A review of redox signaling and the control of MAP kinase pathway in plants.Redox Biol. 2017 Apr;11:192-204. doi: 10.1016/j.redox.2016.12.009. Epub 2016 Dec 9. Redox Biol. 2017. PMID: 27984790 Free PMC article. Review.
-
TALEN-Based HvMPK3 Knock-Out Attenuates Proteome and Root Hair Phenotypic Responses to flg22 in Barley.Front Plant Sci. 2021 Apr 29;12:666229. doi: 10.3389/fpls.2021.666229. eCollection 2021. Front Plant Sci. 2021. PMID: 33995462 Free PMC article.
-
Arabidopsis Iron Superoxide Dismutase FSD1 Protects Against Methyl Viologen-Induced Oxidative Stress in a Copper-Dependent Manner.Front Plant Sci. 2022 Mar 11;13:823561. doi: 10.3389/fpls.2022.823561. eCollection 2022. Front Plant Sci. 2022. PMID: 35360337 Free PMC article.
References
-
- Rodriguez MCS, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010;61:621–649. - PubMed
-
- Šamajová O, Plíhal O, Al-Yousif M, Hirt H, Šamaj J. Improvement of stress tolerance in plants by genetic manipulation of mitogen-activated protein kinases. Biotechnol. Adv. 2013;31:118–128. - PubMed
-
- Andreasson E, Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010;15:106–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

