Identification of a novel recycling sequence in the C-tail of FPR2/ALX receptor: association with cell protection from apoptosis
- PMID: 25326384
- PMCID: PMC4276880
- DOI: 10.1074/jbc.M114.612630
Identification of a novel recycling sequence in the C-tail of FPR2/ALX receptor: association with cell protection from apoptosis
Abstract
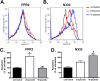
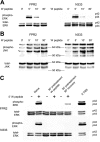
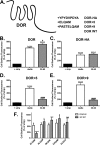
Formyl-peptide receptor type 2 (FPR2; also called ALX because it is the receptor for lipoxin A4) sustains a variety of biological responses relevant to the development and control of inflammation, yet the cellular regulation of this G-protein-coupled receptor remains unexplored. Here we report that, in response to peptide agonist activation, FPR2/ALX undergoes β-arrestin-mediated endocytosis followed by rapid recycling to the plasma membrane. We identify a transplantable recycling sequence that is both necessary and sufficient for efficient receptor recycling. Furthermore, removal of this C-terminal recycling sequence alters the endocytic fate of FPR2/ALX and evokes pro-apoptotic effects in response to agonist activation. This study demonstrates the importance of endocytic recycling in the anti-apoptotic properties of FPR2/ALX and identifies the molecular determinant required for modulation of this process fundamental for the control of inflammation.
Keywords: Apoptosis; Arrestin; Cell Sorting; Endocytosis; G-protein-coupled Receptor (GPCR); Receptor Recycling.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
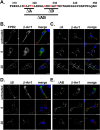



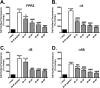

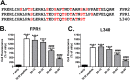
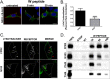
Similar articles
-
GRK5 regulates endocytosis of FPR2 independent of β-arrestins.J Biol Chem. 2025 Feb;301(2):108112. doi: 10.1016/j.jbc.2024.108112. Epub 2024 Dec 18. J Biol Chem. 2025. PMID: 39706266 Free PMC article.
-
Annexin A1 interaction with the FPR2/ALX receptor: identification of distinct domains and downstream associated signaling.J Biol Chem. 2012 Jul 13;287(29):24690-7. doi: 10.1074/jbc.M112.377101. Epub 2012 May 18. J Biol Chem. 2012. PMID: 22610094 Free PMC article.
-
Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity.FASEB J. 2020 May;34(5):6920-6933. doi: 10.1096/fj.201903206R. Epub 2020 Apr 2. FASEB J. 2020. PMID: 32239559
-
Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways.Pharmacol Ther. 2013 Dec;140(3):280-9. doi: 10.1016/j.pharmthera.2013.07.007. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880288 Review.
-
Recent advances in the design and development of formyl peptide receptor 2 (FPR2/ALX) agonists as pro-resolving agents with diverse therapeutic potential.Eur J Med Chem. 2021 Mar 5;213:113167. doi: 10.1016/j.ejmech.2021.113167. Epub 2021 Jan 12. Eur J Med Chem. 2021. PMID: 33486199 Review.
Cited by
-
Targeting Neutrophils for Promoting the Resolution of Inflammation.Front Immunol. 2022 Mar 16;13:866747. doi: 10.3389/fimmu.2022.866747. eCollection 2022. Front Immunol. 2022. PMID: 35371088 Free PMC article. Review.
-
Basigin-mediated redistribution of CD98 promotes cell spreading and tumorigenicity in hepatocellular carcinoma.J Exp Clin Cancer Res. 2015 Oct 6;34:110. doi: 10.1186/s13046-015-0226-6. J Exp Clin Cancer Res. 2015. PMID: 26437640 Free PMC article.
-
Retromer Opposes Opioid-Induced Downregulation of the Mu Opioid Receptor.bioRxiv [Preprint]. 2024 Dec 2:2024.12.02.626482. doi: 10.1101/2024.12.02.626482. bioRxiv. 2024. PMID: 39677727 Free PMC article. Preprint.
-
GRK5 regulates endocytosis of FPR2 independent of β-arrestins.J Biol Chem. 2025 Feb;301(2):108112. doi: 10.1016/j.jbc.2024.108112. Epub 2024 Dec 18. J Biol Chem. 2025. PMID: 39706266 Free PMC article.
References
-
- Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 - PubMed
-
- Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 - PubMed
-
- Dufton N., Perretti M. (2010) Therapeutic anti-inflammatory potential of formyl-peptide receptor agonists. Pharmacol. Ther. 127, 175–188 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

