The human synaptic vesicle protein, SV2A, functions as a galactose transporter in Saccharomyces cerevisiae
- PMID: 25326386
- PMCID: PMC4246065
- DOI: 10.1074/jbc.C114.584516
The human synaptic vesicle protein, SV2A, functions as a galactose transporter in Saccharomyces cerevisiae
Abstract
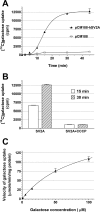
SV2A is a synaptic vesicle membrane protein expressed in neurons and endocrine cells and involved in the regulation of neurotransmitter release. Although the exact function of SV2A still remains elusive, it was identified as the specific binding site for levetiracetam, a second generation antiepileptic drug. Our sequence analysis demonstrates that SV2A has significant homology with several yeast transport proteins belonging to the major facilitator superfamily (MFS). Many of these transporters are involved in sugar transport into yeast cells. Here we present evidence showing, for the first time, that SV2A is a galactose transporter. We expressed human SV2A in hexose transport-deficient EBY.VW4000 yeast cells and demonstrated that these cells are able to grow on galactose-containing medium but not on other fermentable carbon sources. Furthermore, the addition of the SV2A-binding antiepileptic drug levetiracetam to the medium inhibited the galactose-dependent growth of hexose transport-deficient EBY.VW4000 yeast cells expressing human SV2A. Most importantly, direct measurement of galactose uptake in the same strain verified that SV2A is able to transport extracellular galactose inside the cells. The newly identified galactose transport capability of SV2A may have an important role in regulating/modulating synaptic function.
Keywords: Drug Action; Galactose; Levetiracetam; SV2A; Saccharomyces cerevisiae; Sugar Transport; Synapse.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Bajjalieh S. M., Peterson K., Shinghal R., Scheller R. H. (1992) SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257, 1271–1273 - PubMed
-
- Crèvecoeur J., Kaminski R. M., Rogister B., Foerch P., Vandenplas C., Neveux M., Mazzuferi M., Kroonen J., Poulet C., Martin D., Sadzot B., Rikir E., Klitgaard H., Moonen G., Deprez M. (2014) Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol. Appl. Neurobiol. 40, 191–204 - PubMed
-
- Kochubey O., Lou X., Schneggenburger R. (2011) Regulation of transmitter release by Ca2+ and synaptotagmin: insights from a large CNS synapse. Trends Neurosci. 34, 237–246 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

